Krishgen Biosystems is a privately owned manufacturer of therapeutic antibody ELISA kits, HCP detection assays, immunotherapy assay kits, antibodies, proteins, assays for drug development. Krishgen's mission is to provide validated, high quality and high sensitivity assays and reagents worldwide at competitive prices, backed by focused technical support.
Best Seller
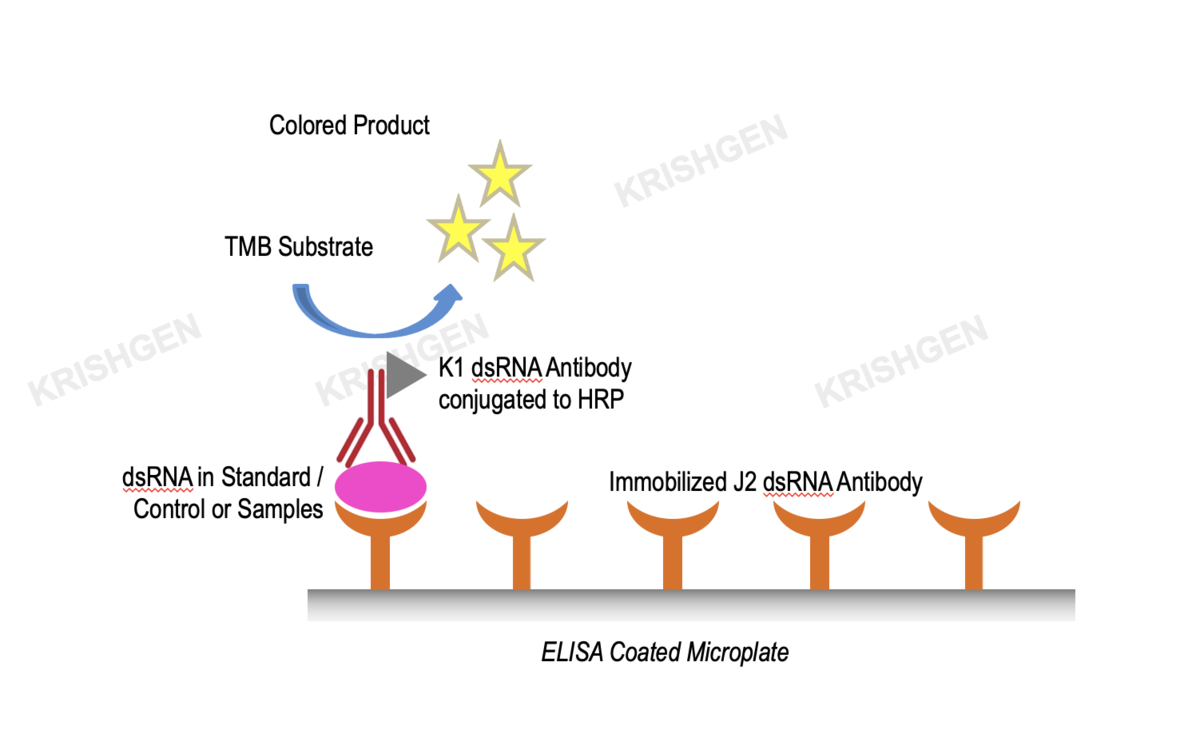
KRIBIOLISA™ Double-Stranded RNA (dsRNA) ELISA (J2 Based)
Robust and sensitive Enzyme Immunoassay for the Qualitative / semi-quantitative screening of double stranded RNA in mRNA based preparations. We recommend using the ELISA to detect viral dsRNAs or large natural or synthetic dsRNAs of non-viral origin in nucleic acid extracts, as well as to detect the presence of undesired dsRNA molecules in artificially synthesized (m)RNA preparations. Serial dilutions of the dsRNA standard (included in the kit) can be used as a positive quantitative control.
Kit features:
- Direct sandwich assay allowing for better specifity
- Pre-Coated plates
- Pre-optimized and validated, ready-to-use and does not require additional validation on user end
- Recovery: 95 - 100%
Cat-No. KBBA56
Description
Introduction: The J2 anti-dsRNA IgG2a monoclonal antibody (Schonborn et al. 1991) has become the gold standard in dsRNA detection. It was used initially for the study of plant viruses, but since the seminal paper of Weber et al. in 2006, where J2 was used to show that all the positive strand RNA viruses tested produced copious amounts of dsRNA in infected cells, this antibody has been used extensively in a wide range of systems, as documented in over 200 scientific publications. J2 can be used to detect dsRNA intermediates of viruses as diverse as Hepatitis C virus, Dengue virus, rhinovirus, Chikungunya virus, Rabies virus, Polio virus, Classic swine fever virus, Brome mosaic virus and many more in cultured cells and also in fixed paraffin-embedded histological samples.
J2 has been used to elucidate how anti-viral responses are initiated, what counter-strategies viruses have adopted to avoid them, and to explore the viral life cylce by enabling ultrastructiural localisation studies of viral nucleic acid replication sites (Welsch et al., 2009 & Knoops et al., 2011). J2 has also been recommended as a diagnostic tool to detect whether an unkown pathogen is bacterial or viral in nature (Richardson et al., 2010). Recently J2 has also been used to monitor the removal of dsRNA from in vitro synthethised mRNA preparations that may have potential use in gene therapy (Kariko et al., 2011). J2 has been used successfully in various immunocapture methods, such as ELISA.
Assay Principle: The KRIBIOLISA Double-Stranded RNA (dsRNA) ELISA employs the quantitative sandwich enzyme immunoassay technique. It is based on the use of two double-stranded RNA (dsRNA)-specific monoclonal antibodies which allows sensitive and selective detection of dsRNA molecules (>=40 bp), independent of their nucleotide composition and sequence. Antibodies to dsRNA (J2) are pre-coated onto microwells. Samples and standards are pipetted into microwells and are bound by the capture antibody. Then, a HRP (horseradish peroxidase) conjugated Anti-dsRNA (K1) is pipetted and incubated. After washing microwells in order to remove any non-specific binding, the ready to use substrate solution (TMB) is added to microwells and color develops proportionally to the amount of dsRNA in the sample. Color development is then stopped by addition of stop solution. Absorbance is measured at 450 nm.
Assay Procedure:
- Bring all reagents to Room Temperature prior to use. It is strongly recommended that all Controls and samples should be run in duplicates or triplicates.
- Add 100ul of prepared Positive Control/Standards and Samples in their respective wells.
- Seal the plate and Incubate at 37*C for 120 minutes.
- Aspirate and wash the plate 4 times with Wash Buffer (1X) and blot residual buffer by firmly tapping plate upside down on absorbent paper. Wipe of any liquid from the bottom outside of the microtiter wells as any residue can interfere in the reading step.
- Add 100ul of dsRNA-specific K1 Detection:HRPConjugate to all the wells.
- Seal the plate and Incubate at 37*C for 60 minutes.
- Repeat the wash step (4).
- Add 100 ul of TMB substrate in each well.
- Incubate the microplate for 30 minutes at RT in dark. DO NOT SHAKE or else it may result in higher backgrounds and worse precision. Positive wells should turn bluish in color.
- Pipette out 100 ul of Stop Solution. Wells should turn from blue to yellow in color.
- Read the absorbance at 450 nm with a microplate reader.
KRIBIOLISA - ELISA kits for bioprocess contamination detection
Product impurities and host cell proteins (HCPs) are inevitable during biopharmaceutical manufacturing, regardless of whether biopharmaceuticals are produced by recombinant fermentation or extracted from natural sources. Krishgen Biosciences offers a variety of ELISA kits validated for impurities of downstream contaminants.
- Measure Host Cell Protein and Protein A populations in sample
- Use of polyclonal antibodies ensures a high degree of specific immunoreactivity
- Ready-made kits for easy set-up and use
- All components are included
| Cat-No. | Kit | Size |
|---|---|---|
| KBBP01 | KRIBIOLISA™ E.coli HCP ELISA | 96 wells |
| KBBP02 | KRIBIOLISA™ Pichia Pastoris HCP ELISA | 96 wells |
| KBBP03 | KRIBIOLISA™ CHO HCP ELISA | 96 wells |
| KBBP08 | KRIBIOLISA™ Human IgG ELISA | 96 wells |
| KBBP03 | KRIBIOLISA™ Protein A ELISA | 96 wells |
| KBBR11 | KRIBIOLISA™ E.coli Host Cell DNA Kit | 96 wells |
| KBBR02 | KRIBIOLISA™ CHO Host Cell DNA Kit | 96 wells |
KRIBIOLISA - ELISA kits for detecting biosimilars and biologics
The KRIBIOLISA range of Therapeutic Drug Monitoring ELISA kits can be used for efficient biological drug testing by accurately monitoring serum trough levels and the presence of anti-drug antibodies respectively. Our kits are also CE IVD marked for diagnostic use.
KRIBIOLISA kits can be used in various clinical and research settings:
- To measure dosage response and required adjustment of individual patients, by measuring both drug levels and immunogenicity
- To design and optimise new biopharmaceuticals
- Preclinical safety assessment studies of mAbs
- Biosimilar studies (pharmacokinetics and immunogenicity)
- Clinical trials
Advantages of KRIBIOLISA kits
- Based on monoclonal antibodies for better sensitivity
- Sandwich ELIS Adesign and specific antibodies allow for a robust ELISA with low cross reactivity
- Standards calibrated against NIBSC and commercially available innovator/patented mAb pharmaceuticals
- Available in quantitative formats