What are biosimilars?
Biologicals (or biologics) are antibodies or proteins which are typically produced in animal cells, yeast, or bacteria and which have been approved for therapeutical use in humans. Their structure, production and formulation is strictly defined and must be approved by a regulatory body, such as the FDA or EMA. Therapeutic antibodies in particular are a rapidly evolving class of drugs that has been gaining ground over small molecules for the treatment of cancer, autoimmune diseases, and reversal of drug effects. Their success in the clinic stems from their ability to engage specific targets (i.e. antigens) through their antigen-binding region and, at the same time, activate the organism’s immune response through their crystallizable region (Fc).
A biosimilar is structurally identical to its parent biological and has a similar therapeutic effect in humans. Like a biological, it must be produced under strictly defined conditions and must be approved by a regulatory body. A biosimilar shares its sequence with the parent biological, but its potency, stability and side-effects may be different due to the inherent complexity of the manufacturing process. This is markedly different from generics, which are chemically defined substances and which normally do not differ either in composition or formulation from the parent substance.
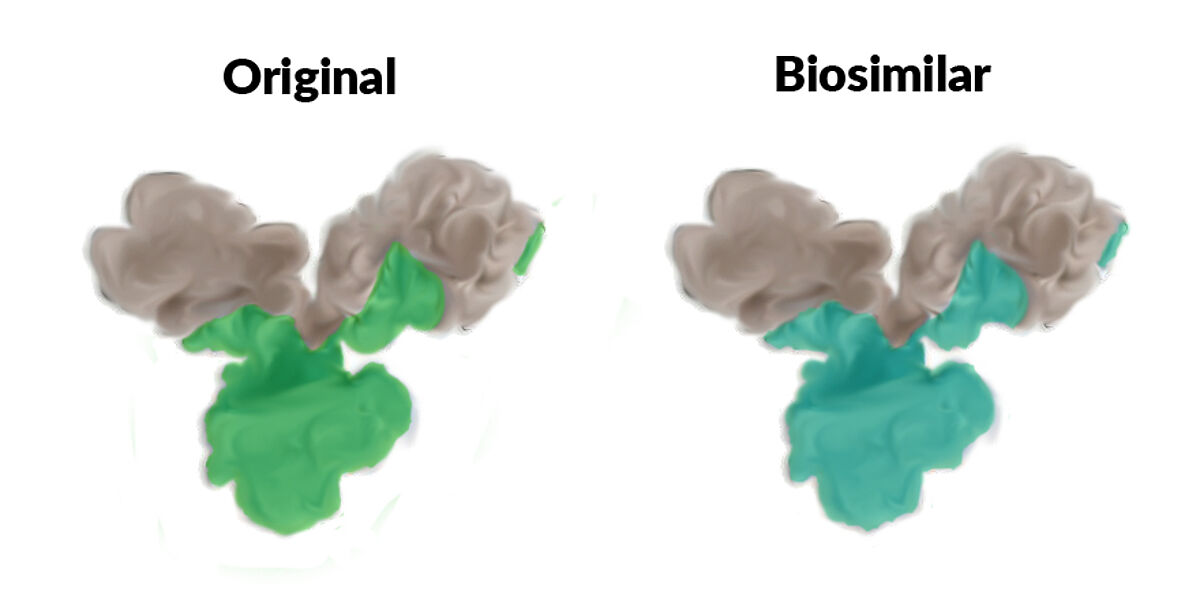
When using biosimilars in an R&D setting it is therefore important to carefully select the biosimilar to be as close to the parent biological as possible, as they can vary considerably in terms of sequence/framework fidelity, purity and activity.
R&D use biosimilar make it possible to study the biological effects of a drug without needing to source expensive pharmaceutical-grade therapeutics. They can be used for many research applications, including functional assays, flow cytometry, ELISA, Immunohistochemistry, pharmacokinetic assays, and others.
R&D use of biosimilar antibodies
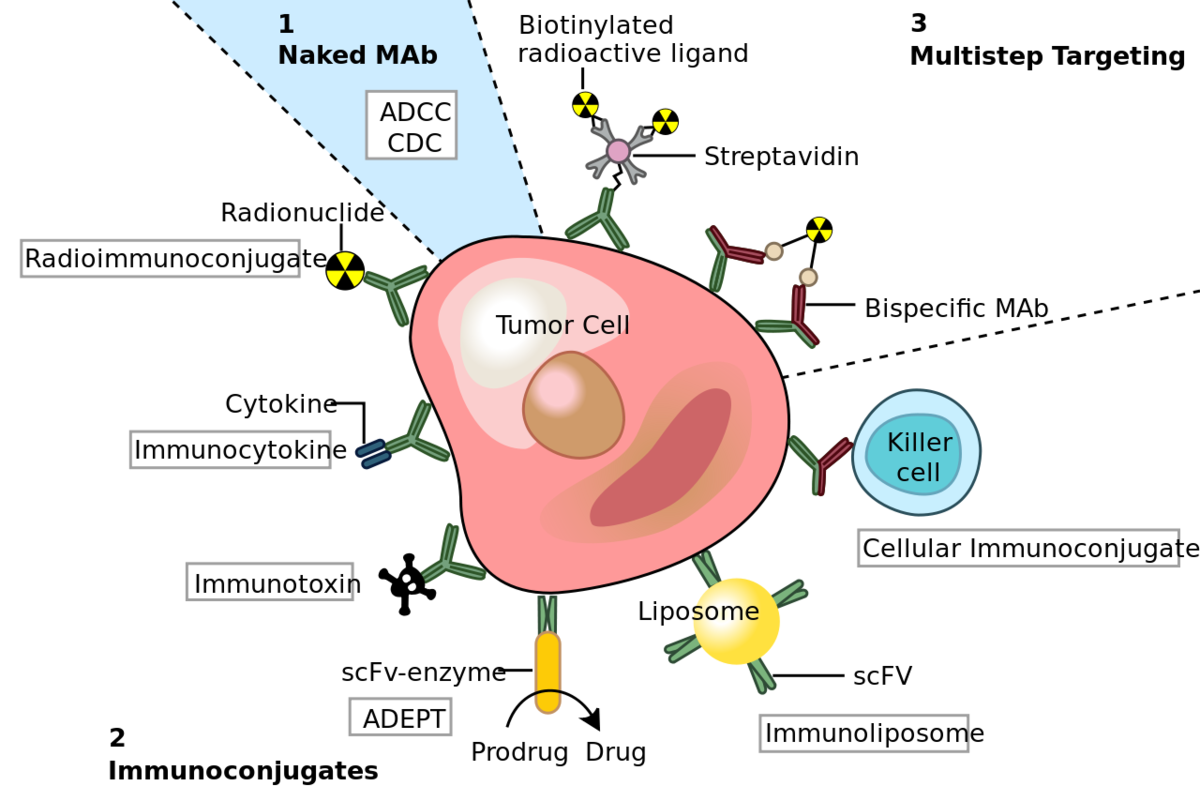
Biosimilars can be used in a wide range of applications. For instance, they can block specific pathogens or pathophysiological pathways. These therapeutic antibodies are termed neutralizing. Antibodies can also be designed to recruit specific cytotoxic cells (i.e. macrophages and natural killer cells), triggering the so-called antibody-dependent cell-mediated cytotoxic (ADCC) activity. Additionally, therapeutic antibodies can trigger a cascade of complement proteins, leading to lysis of the targeted cells. This mechanism is known as the complement-dependent cytotoxic (CDC) activity.
Most therapeutic antibodies, especially the ones developed for cancer, can trigger more than one type of immune response (neutralizing, ADCC, an CDC) in patients. The type of effector function an antibody can trigger heavily depends on the glycosylation patterns of the Fc regions. Glycans can cause significant differences in conformation, which results in changes in antibody affinity and the ability to activate the complement system. For this reason, the efficiency of therapeutic antibodies depends on the recombinant expression system used to produce them. For many years, CHO (Chinese hamster ovary) cells have been the most important mammalian cell lines used in therapeutic antibody production. They are prized for their ability to produce mAbs with human-like glycosylation, crucial to trigger essential effector responses in the human organism.
Bio X Cell
Bio X Cell InVivoSIM™ Biosimilar Antibodies are research-grade biosimilar monoclonal antibodies. Each biosimilar antibody is produced using recombinant technology and incorporates the same variable region sequence as the original pharmaceutical drug. InVivoSIM™ biosimilar antibodies are offered with the same purity and in vivo formulations that Bio X Cell is known for. They are ultra-pure and free of preservatives, stabilizers, and carrier proteins, making them ideal for in vivo applications including drug efficacy studies in humanized mouse models.
- QC tested for purity using SDS-PAGE, aggregate level < 5% of total protein
- Validated for antigen binding by immunoblot
- Ultra-low endotoxin levels ≤ 1EU/mg
- Pathogen Free, conform to IACUC and Animal Facility requirements

Absolute Antibody
Absolute Antibody research-grade biosimilars are research tools for the study and evaluation of biological processes without the need to source and purchase costly therapeutic-grade biologics. Absolute Antibody research-grade biosimilars can be used as controls to benchmark novel biotherapeutics (biologics) for pre-clinical lead identification or for the development of potency assays.
- Free of excipients and/or additives found in the therapeutic formulations
- Require fewer extra controls in an experimental setting
- Original human format, or available with mouse, rabbit, rhesus monkey and cynomolgus monkey constant domains
- All research-grade biosimilars are supplied in PBS buffer with preservative (0.02% Proclin 300)
- Endotoxin is < 1.0 EU/mg as determined by the LAL method
- Purity is > 96% as determined by SDS-PAGE

| Cat-No. | Item | Size | Price (CHF) |
|---|---|---|---|
| Ab00103-10.0 | Anti erbB-2 (Her-2/neu) [4D5-8 (trastuzumab)], IgG1 | 200 ug | 645.00 |
| Ab00103-10.3 | Anti erbB-2 (Her-2/neu) [4D5-8 (trastuzumab)], IgG1, Fc Silent™ | 200 ug | 645.00 |
| Ab00126-10.0 | Anti CD20 [10F381 (rituximab)], IgG1 | 200 ug | 645.00 |
| Ab00126-10.3 | Anti CD20 [10F381 (rituximab)], IgG1, Fc Silent™ | 200 ug | 645.00 |
| Ab00146-10.0 | Anti TNF alpha [cA2 (Infliximab)], IgG1 | 200 ug | 645.00 |
| Ab00146-10.3 | Anti TNF alpha [cA2 (Infliximab)], IgG1, Fc Silent™ | 200 ug | 645.00 |
| Ab00279-10.0 | Anti EGFR [C225 (Cetuximab)], IgG1 | 200 ug | 645.00 |
| Ab00279-10.2 | Anti EGFR [C225 (Cetuximab)], IgG1, Aglycosylated | 200 ug | 645.00 |
| Ab00279-10.3 | Anti EGFR [C225 (Cetuximab)], IgG1, Fc Silent™ | 200 ug | 645.00 |
| Ab00282-10.0 | Anti CD41 [7E3 (Abciximab)], IgG1 | 200 ug | 645.00 |
| Ab00282-10.3 | Anti CD41 [7E3 (Abciximab)], IgG1, Fc Silent™ | 200 ug | 645.00 |
| Ab00283-10.0 | Anti CD33 [hP67.6 (Gemtuzumab)], IgG1 | 200 ug | 645.00 |
| Ab00447-10.0 | Anti CD40L [hu5c8 (Ruplizumab)], IgG1 | 200 ug | 645.00 |
| Ab00447-10.3 | Anti CD40L [hu5c8 (Ruplizumab)], IgG1, Fc Silent™ | 200 ug | 645.00 |
| Ab00535-10.0 | Anti CD11a [hu1124 (Efalizumab)], IgG1 | 200 ug | 645.00 |
| Ab00535-10.3 | Anti CD11a [hu1124 (Efalizumab)], IgG1, Fc Silent™ | 200 ug | 645.00 |
| Ab00536-10.0 | Anti OX40L [R4930 (Oxelumab)], IgG1 | 200 ug | 645.00 |
| Ab00536-10.3 | Anti OX40L [R4930 (Oxelumab)], IgG1, Fc Silent™ | 200 ug | 645.00 |
| Ab00717-10.0 | Anti IgE [huMaE11 (Omalizumab)], IgG1 | 200 ug | 645.00 |
| Ab00717-10.3 | Anti IgE [huMaE11 (Omalizumab)], IgG1, Fc Silent™ | 200 ug | 645.00 |
| Ab00718-10.0 | Anti TNF alpha [D2E7 (Adalimumab)], IgG1 | 200 ug | 645.00 |
| Ab00718-10.3 | Anti TNF alpha [D2E7 (Adalimumab)], IgG1, Fc Silent™ | 200 ug | 645.00 |
| Ab00722-10.0 | Anti IL-12/23 [ABT-874 (Briakinumab)], IgG1 | 200 ug | 645.00 |
| Ab00722-10.17 | Anti IL-12/23 [ABT-874 (Briakinumab)], IgG1 | 200 ug | 645.00 |
| Ab00722-10.3 | Anti IL-12/23 [ABT-874 (Briakinumab)], IgG1, Fc Silent™ | 200 ug | 645.00 |
| Ab00723-10.0 | Anti EGFR domain III [h-R3 (Nimotuzumab)], IgG1 | 200 ug | 645.00 |
| Ab00723-10.3 | Anti EGFR domain III [h-R3 (Nimotuzumab)], IgG1, Fc Silent™ | 200 ug | 645.00 |
| Ab00728-10.0 | Anti CD22 [hL22 (Epratuzumab)], IgG1 | 200 ug | 645.00 |
| Ab00728-10.3 | Anti CD22 [hL22 (Epratuzumab)], IgG1, Fc Silent™ | 200 ug | 645.00 |
| Ab00729-10.0 | Anti RSV [RSHZ19 (Felvizumab)], IgG1 | 200 ug | 645.00 |
| Ab00729-10.3 | Anti RSV [RSHZ19 (Felvizumab)], IgG1, Fc Silent™ | 200 ug | 645.00 |
| Ab00736-10.0 | Anti CD80 [IDEC-114 (Galiximab)], IgG1 | 200 ug | 645.00 |
| Ab00736-10.3 | Anti CD80 [IDEC-114 (Galiximab)], IgG1, Fc Silent™ | 200 ug | 645.00 |
| Ab00737-10.0 | Anti IL-6 receptor [rhPM-1 (Tocilizumab)], IgG1 | 200 ug | 645.00 |
| Ab00737-10.3 | Anti IL-6 receptor [rhPM-1 (Tocilizumab)], IgG1, Fc Silent™ | 200 ug | 645.00 |
| Ab00740-10.0 | Anti DR5 [PRO95780 (Drozitumab)], IgG1 | 200 ug | 645.00 |
| Ab00740-10.3 | Anti DR5 [PRO95780 (Drozitumab)], IgG1, Fc Silent™ | 200 ug | 645.00 |
| Ab00773-10.0 | Anti Lymphotoxin alpha [MLTA3698A (Pateclizumab)], IgG1 | 200 ug | 645.00 |
| Ab00773-10.3 | Anti Lymphotoxin alpha [MLTA3698A (Pateclizumab)], IgG1, Fc Silent™ | 200 ug | 645.00 |
| Ab01678-10.0 | Anti CD154 [IDEC-131 (Toralizumab)], IgG1 | 200 ug | 645.00 |
| Ab01678-10.3 | Anti CD154 [IDEC-131 (Toralizumab)], IgG1, Fc Silent™ | 200 ug | 645.00 |
| Ab02032-10.0 | Anti Lipoteichoic acid [BSYX-A110 (Pagibaximab)], IgG1 | 200 ug | 645.00 |
| Ab02032-10.3 | Anti Lipoteichoic acid [BSYX-A110 (Pagibaximab)], IgG1, Fc Silent™ | 200 ug | 645.00 |
| Ab02245-10.0 | Anti CCL2 [CNTO 888 (Carlumab)], IgG1 | 200 ug | 645.00 |
| Ab02245-10.3 | Anti CCL2 [CNTO 888 (Carlumab)], IgG1, Fc Silent™ | 200 ug | 645.00 |
| Ab02329-10.0 | Anti CD4 [6G5 (Zanolimumab)], IgG1 | 200 ug | 645.00 |
| Ab02329-10.3 | Anti CD4 [6G5 (Zanolimumab)], IgG1, Fc Silent™ | 200 ug | 645.00 |
| Ab03058-10.0 | Anti VEGFR2 [IMC-1121B (Ramucirumab)], IgG1 | 200 ug | 645.00 |
| Ab03058-10.3 | Anti VEGFR2 [IMC-1121B (Ramucirumab)], IgG1, Fc Silent™ | 200 ug | 645.00 |
| Ab03369-10.0 | Anti HLA-DR [1D10 (Hu1D10, Apolizumab)], IgG1 | 200 ug | 645.00 |
| Ab03369-10.3 | Anti HLA-DR [1D10 (Hu1D10, Apolizumab)], IgG1, Fc Silent™ | 200 ug | 645.00 |
ProteoGenix
- Over 1000 biosimilars in different formulations
- Coverage of popular targets, such as IL's, CTLA-4, CD30, PD1, TNF and others
- Isotope-labeled biosimilars for MS applications
- Applications: WB, ELISA, MS

ACROBiosystems
ACROBiosystems has developed high quality target antigen proteins and Fc receptor proteins to facilitate biosimilar research.
- Validated with reference innovator drugs
- High batch-to-batch consistency
- High stability & high activity
- High purity & high quality

| Cat-No. | Item | Size | Price (CHF) |
|---|---|---|---|
| CD0-M36-100ug | Rituximab biosimilar-Research Grade (MALS verified) | 100 ug | 187.00 |
| ADB-Y23b-100ug | Anti Adalimumab Antibody (AY23b) (recommended for PK/PD) | 100 ug | 374.00 |
| RIB-Y35c-100ug | Anti Rituximab Antibody (MALS verified) | 100 ug | 374.00 |
| RIB-FY35c-25ug | FITC-Labeled Anti Rituximab Antibody, Mouse IgG1 | 25 ug | 399.00 |
| RIB-Y36-100ug | Anti Rituximab Antibody (AY36) (recommended for ADA assay) | 100 ug | 374.00 |
| RIB-Y37-100ug | Anti Rituximab Antibody (AY37) (recommended for PK/PD) | 100 ug | 374.00 |
| TRB-Y1b-100ug | Anti Trastuzumab Antibody (AY1b) (recommended for PK/PD) | 100 ug | 374.00 |
| CEB-Y31-100ug | Anti Cetuximab Antibody (AY31) (Non-Neutralizing) | 100 ug | 374.00 |
| CEB-Y28-100ug | Anti Cetuximab Antibody (AY28) | 100 ug | 374.00 |
| ADB-Y19-100ug | Anti Adalimumab Antibody (AY19) | 100 ug | 374.00 |
| BEB-Y9-100ug | Anti Bevacizumab Antibody (AY9) (recommended for ADA assay) | 100 ug | 374.00 |
| BEB-Y12-100ug | Anti Bevacizumab Antibody (AY12) (recommended for neutralizing assay) | 100 ug | 374.00 |
| CEB-Y27-100ug | Anti Cetuximab Antibody (AY27) (recommended for ADA assay) | 100 ug | 374.00 |
| CEB-Y29-100ug | Anti Cetuximab Antibody (AY29) (recommended for PK/PD) | 100 ug | 396.00 |
| CEB-BY31-25ug | Biotinylated Anti Cetuximab Antibody (AY31) (recommended for PK/PD) | 25 ug | 459.00 |
| BEB-BY13-25ug | Biotinylated Anti Bevacizumab Antibody (AY13) (recommended for PK/PD) | 25 ug | 435.00 |
| BEB-Y10-100ug | Anti Bevacizumab Antibody (AY10) (MALS verified, recommended for PK/PD) | 100 ug | 374.00 |
| TRB-Y5b-100ug | Anti Trastuzumab Antibody, Mouse IgG1 (6F2F7-2) (recommended for PK/PD) | 100 ug | 374.00 |
KRIBIOLISA - ELISA kits for detecting biosimilars and biologics
The KRIBIOLISA range of Therapeutic Drug Monitoring ELISA kits can be used for efficient biological drug testing by accurately monitoring serum trough levels and the presence of anti-drug antibodies respectively. Our kits are also CE IVD marked for diagnostic use.
KRIBIOLISA kits can be used in various clinical and research settings:
- To measure dosage response and required adjustment of individual patients, by measuring both drug levels and immunogenicity
- To design and optimise new biopharmaceuticals
- Preclinical safety assessment studies of mAbs
- Biosimilar studies (pharmacokinetics and immunogenicity)
- Clinical trials
Advantages of KRIBIOLISA kits
- Based on monoclonal antibodies for better sensitivity
- Sandwich ELIS Adesign and specific antibodies allow for a robust ELISA with low cross reactivity
- Standards calibrated against NIBSC and commercially available innovator/patented mAb pharmaceuticals
- Available in quantitative formats
SelleckChem
SelleckChem offers more than 50 different biosimilars.
- Highly purified
- Proven activity
- Available in different standard sizes and bulk sizes

Antibodies.com
Antibodies.com supplies high-quality biological reagents to life science researchers at cost-effective prices. With currently over 600 biosimilars in their catalogue, they support life scientists worldwide by supplying the same high-quality products as the industry leading suppliers but at more affordable prices.

Further information
For further information on biosimilars in Switzerland, please visit biosimilar.ch.