Structural determination with Cryo-EM
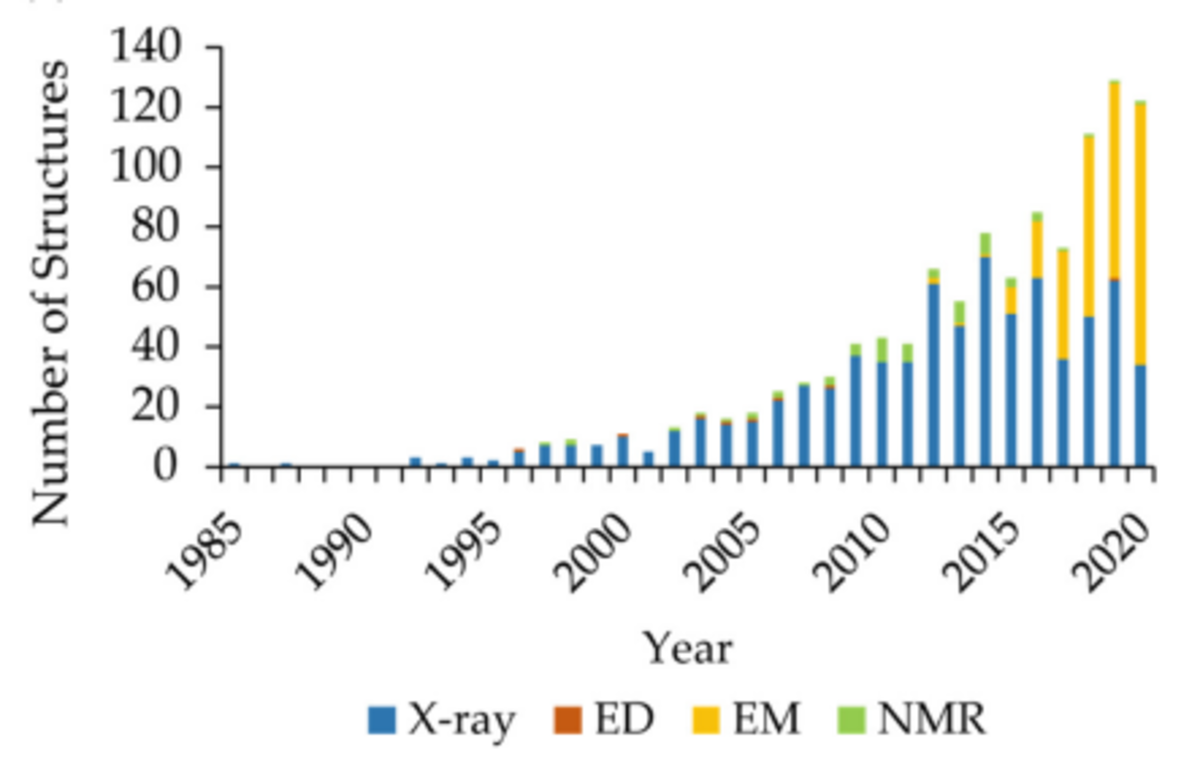
Cryo Electron Microscopy (CryoEM) has long held potential for the study of larger proteins and complexes, especially membrane proteins (MPs), as the technique does not require crystallisation of proteins. Instead, for CryoEM the protein solution is rapidly frozen into a thin, vitrified layer and proteins are imaged as single particles. However, the utility of CryoEM for structural determination, especially for drug discovery, has historically been limited by the resolution that could be achieved and the minimum size of structures that could be imaged. In recent years significant technological advances have been made, including high quantum efficiency, direct electron detectors coupled with improved software tools and increased computing power. These improvements have driven what is commonly referred to as the ‘resolution revolution’[2] dramatically increasing the use of CryoEM for structural biology. Single particle Cryo-EM has now displaced protein crystallography to become the dominant method for solving the molecular structure of MPs [Figure 1].
Challenges of determining membrane protein structure
Membrane proteins (MPs) are the primary class of protein targets for drug discovery as they perform a wide range of critical functions, both at the cell surface and within intra-cellular membranes, making up an estimated 20-30% of the genes in almost all known genomes [1]. The dysfunction of membrane proteins can be extremely detrimental making the study of them fundamental to the understanding of the molecular basis for diseases and to the development of therapeutics. However, MPs remain incredibly challenging to work with due to, amongst other things, low abundance, poor stability, and poor solubility.
Additionally, the vitrification process to prepare samples for CryoEM can also be problematic due to proteins interacting with the grid material or air interface which can cause unwanted effects such as adsorption, aggregation, and denaturation/degradation. It is common to screen additives, such as detergents and lipids to optimise the vitrification process. To this end, researchers working using CryoEM, especially with membrane proteins, require a toolbox of highly reliable and reproducible reagents to maximise their return on time and limited sample. For more than 30 years Anatrace have been trusted by the community to provide high purity detergents, lipids and other reagents. In this article we bring together a selection of Anatrace reagents from which you can build your toolbox for discovery.
Removal of membrane proteins from the lipid membrane
One of the key challenges when working with MPs is the requirement to remove the protein from the lipid membrane in which it is inherently stable. Detergents are still among the most common tools used and are employed in three distinct steps towards obtaining an MP structure, specifically solubilisation, purification and structure determination. Choosing the right detergent(s) to use at each step is critical when trying to purify an MP for crystallisation or cryo-EM.
However, whilst detergents have been an effective way to solubilise MPs, in certain cases their use can lead to a loss of protein–protein and protein–lipid interactions, potentially destabilising the MP.
Amphipols are short amphipathic polymers that can substitute for detergents at the transmembrane surface of MPs and, thereby keep them soluble in detergent free aqueous solutions. APol-trapped MPs are, as a rule, more stable biochemically than their detergent-solubilized counterparts. Amphipols have been shown to be effective tools for cryo-EM sample delivery with the most widely used being A8-35.
In addition to detergents and amphipols a number of MP structures have been determined by reconstituting the MP of interest into lipodiscs which provide the protein with a lipid bilayer environment, which can aide protein structure and activity [5]. Styrene maleic acid (SMA), SMA derivatives and poly-diisobutylene-co-maleic acid (DIBMA) are available for MP solubilisation and enable direct solubilisation of an MP into a lipodisc without the requirement for detergent.
Anatrace also offer a lipid mix for Nanodisc/Saposin reconstitution, Cat# P516:P416:P616, which composes of a 3:1:1 ratio of POPC:POPE:POPG. This lipid mixture has been successfully used to determine the structure of channel proteins, including NOMPC, TRPML1, TRPM4 and Kv1.2-2.1 paddle chimera. If you need another lipid mixture for your reconstitution then just ask as Anatrace can prepare a mixture to meet your needs.
Detergents
Anatrace had led the way in providing high-purity detergents and offers a wide range of detergent classes as listed below. They offer the largest portfolio in the industry with more than 50 unique detergents.
- Amine Oxides
- CYMAL
- Glucosides
- HEGA & MEGA
- Maltosides
- Mannitol-based Amphiphiles
- Melibiosides
- NG Class
- Thio Glucosides & Maltosides
- Trehalosides
The below selection of detergents has proven to have broad applicability [4][6] for working with membrane proteins and for determining their structures. It is worth noting that GDN, Lipid Nanodiscs and Amphipols have particular application for CryoEM [6]
Name: DDM - n-Dodecyl-β-D-Maltopyranoside
Formula: C24H46O11
Cat No: D310
Pack Sizes: 1 g, 5 g, 25 g
Name: 10:1 DDM/CHS Pre-Made Solution
Formula: Dodecyl Maltoside (D310) and Cholesteryl Hemisuccinate (CH210)
Cat No: D310-CH210
Pack Sizes: 1 ml, 10 ml, 50 ml
Name: GDN - Synthetic drop-in substitute for Digitonin
Formula: C56H92O25
Cat No: GDN101
Pack Sizes: 500 mg, 1 g, 5 g, 25 g
Name: LMNG - Lauryl Maltose Neopentyl Glycol
Formula: C47H88O22
Cat No: NG310
Pack Sizes: 1 g, 5 g, 25 g
Name: DM - n-Decyl-β-D-Maltopyranoside
Formula: C22H42O11
Cat No: D322
Pack Sizes: 1 g, 5 g, 25 g
Name: α-DDM - n-Dodecyl-α-D-Maltopyranoside, Anagrade
Formula: C24H46O11
Cat No: D310HA
Pack Sizes: 1 g, 5 g, 25 g
Name: OG - n-Octyl-β-D-Glucopyranoside
Formula: C14H28O6
Cat No: O311
Pack Sizes: 1 g, 5 g, 25 g
Name: n-Nonyl β-D-Glucopyranoside
Formula: C15H30O6
Cat No: N324
Pack Sizes: 1 g, 5 g, 25 g
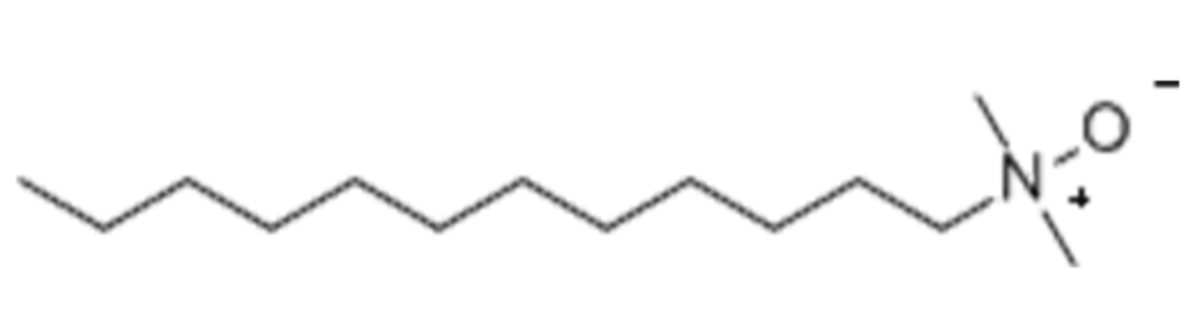
Name: LDAO - Lauryl dimethylamine-N-Oxide
Formula: C14H31NO
Cat No: D360
Pack Sizes: 1 g, 5 g, 25 g
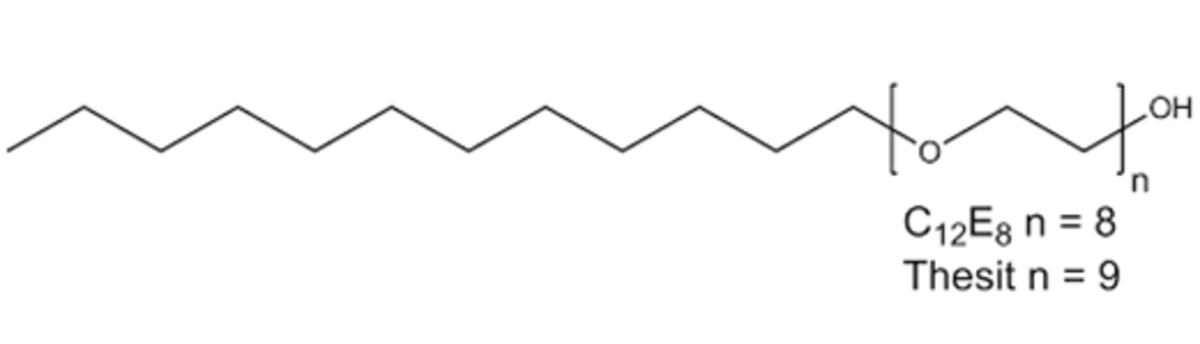
Name: C12E8 - Polyoxyethylene 8 (9) dodecyl Ether Octaethylene Glycol Monododecyl Ether
Formula: C28H58O9
Cat No: O330
Pack Sizes: 4 ml, 2x10 ml, 10x10 ml
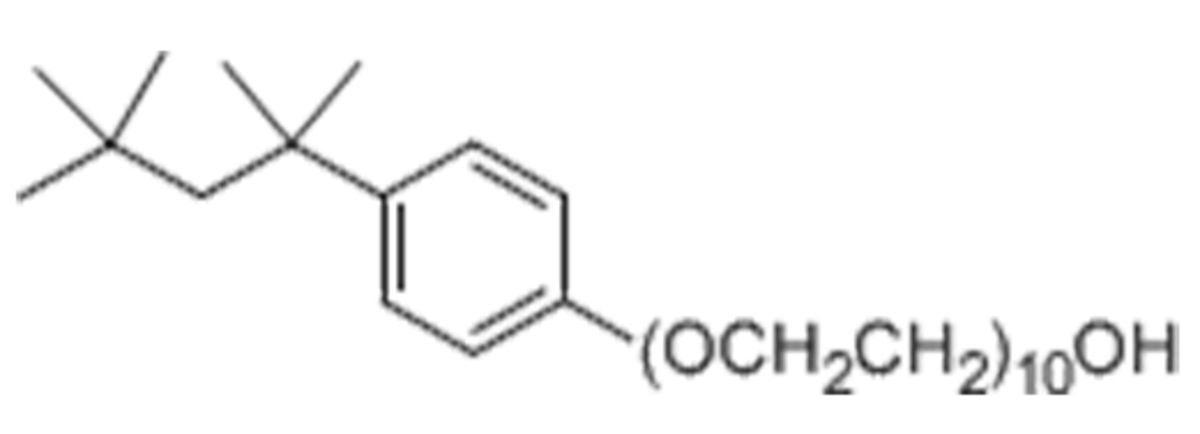
Name: Thesit - Polyoxyethylene 9 dodecyl Ether
Formula: (C2H4O)nC12H26O n ~ 9
Cat No: APO129
Pack Sizes: 5x10 ml, 50x1 ml, 10x10 ml, 500 ml
Name: UDM - n-Undecyl-β-D-Maltopyranoside
Formula: C23H44O11
Cat No: U300
Pack Sizes: 1 g, 5 g, 25 g
Name: Triton X-100
Formula: t-Oct-C6H4-(OCH2CH2)XOH, x = 9-10
Cat No: T1001
Pack Sizes: 500 ml, 1 gallon
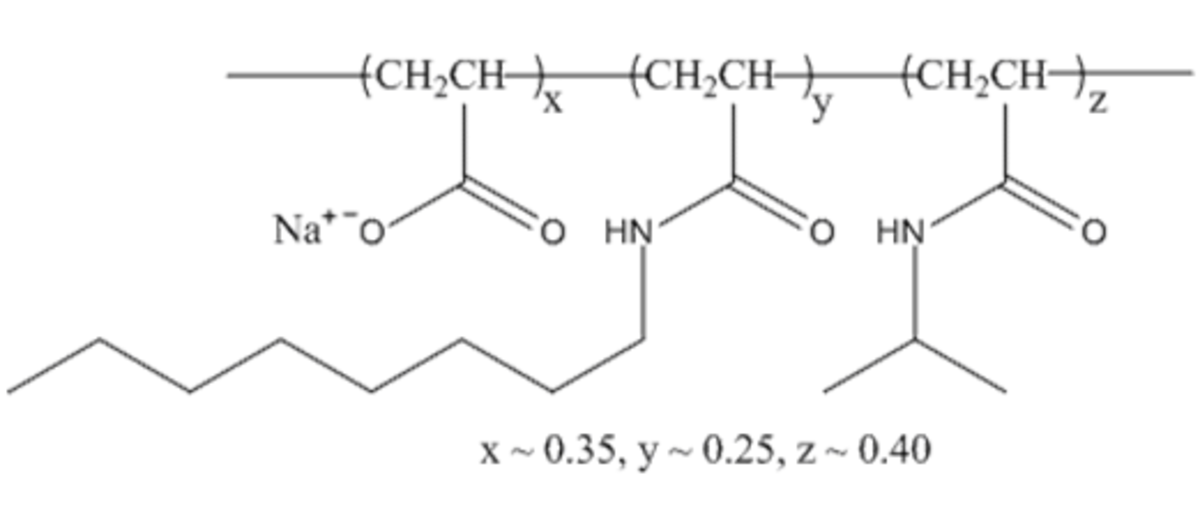
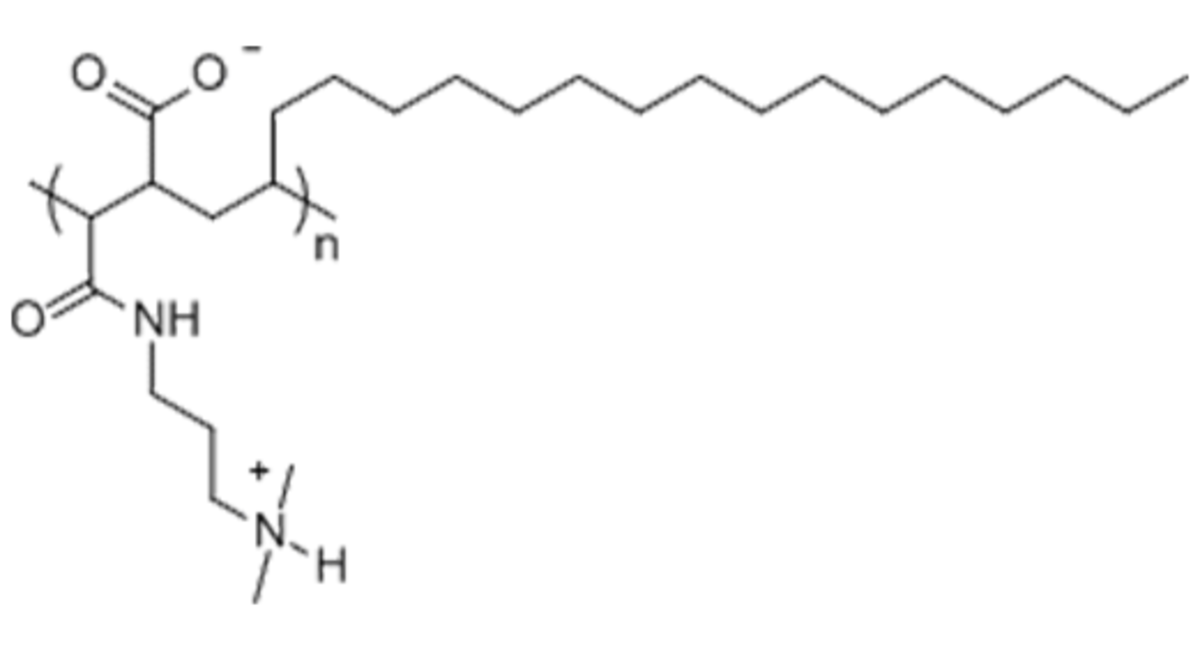
Name: A8-35
Formula: (C6.2H10.3O1.35N0.65Na0.35)~72
Cat No: A835
Pack Sizes: 50 mg, 100 mg, 500 mg
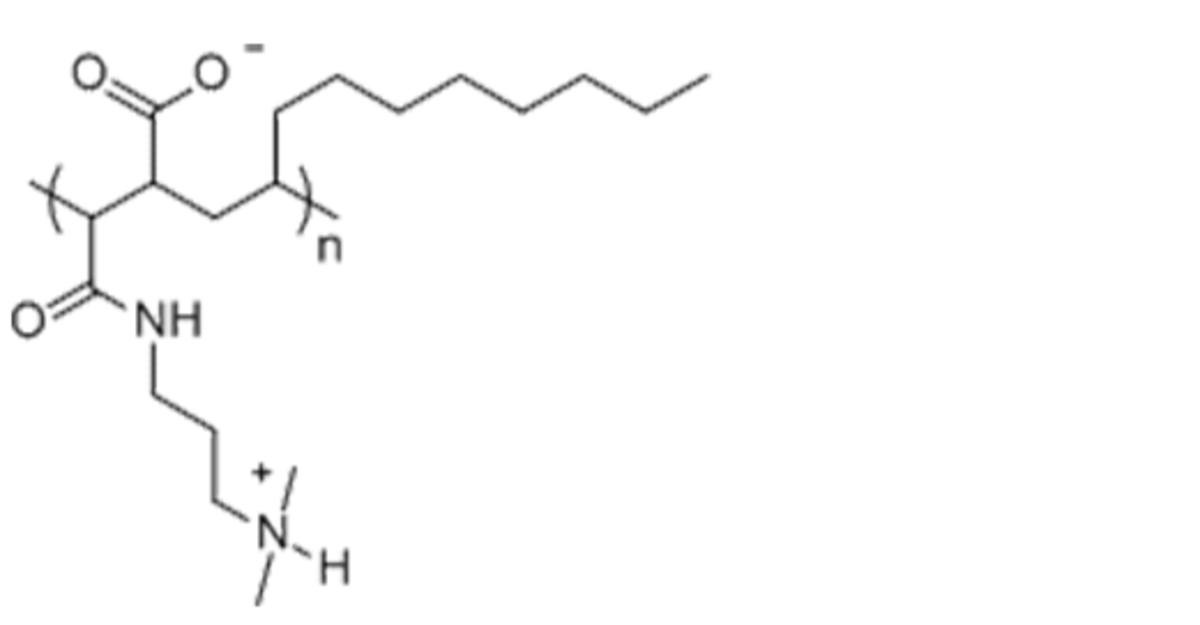
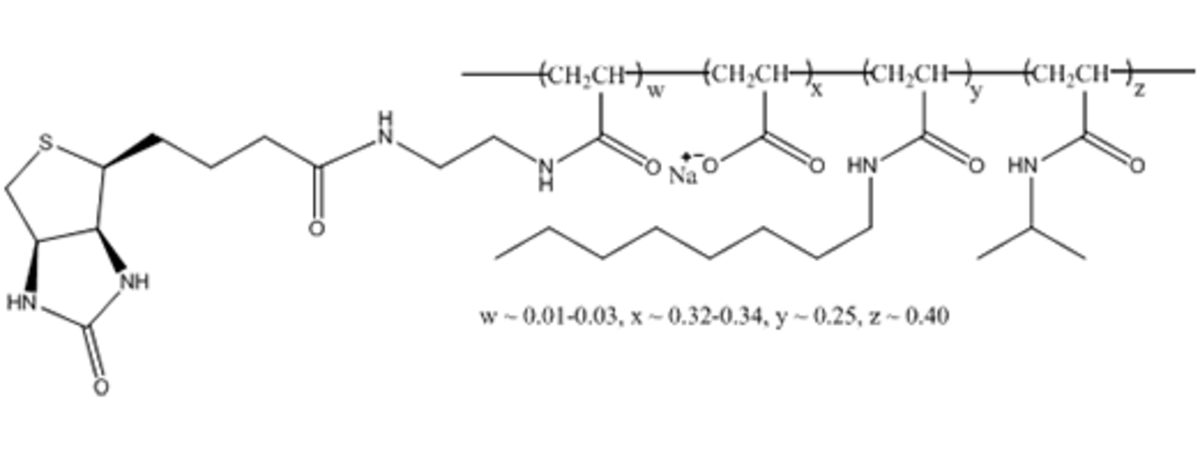
Name: PMAL-C8 - Poly (Maleic Anhydride-alt-1-Decene) substituted with 3-(Dimethylamino) Propylamine
Formula: (C19H36O3N2)n
Cat No: P5008
Pack Sizes: 1 g, 5 g
Name: PMAL-C12 - Poly (Maleic Anhydride-alt-1-Tetradecene) substituted with 3-(Dimethylamino) Propylamine
Formula: (C23H44O3N2)n
Cat No: P5012
Pack Sizes: 1 g, 5 g
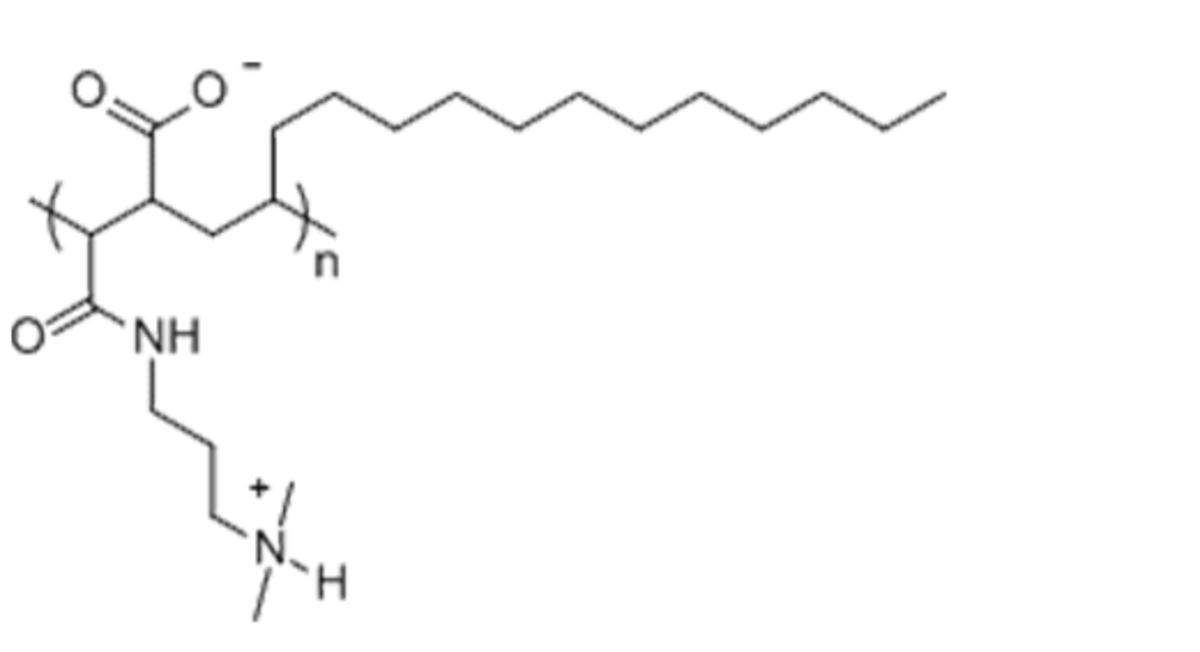
Name: PMAL-C16 - Poly (Maleic Anhydride-Alt-1-Octadecene) substituted with 3-(Dimethylamino) Propylamine
Formula: (C27H52O3N2)n
Cat No: P5016
Pack Sizes: 1 g, 5 g
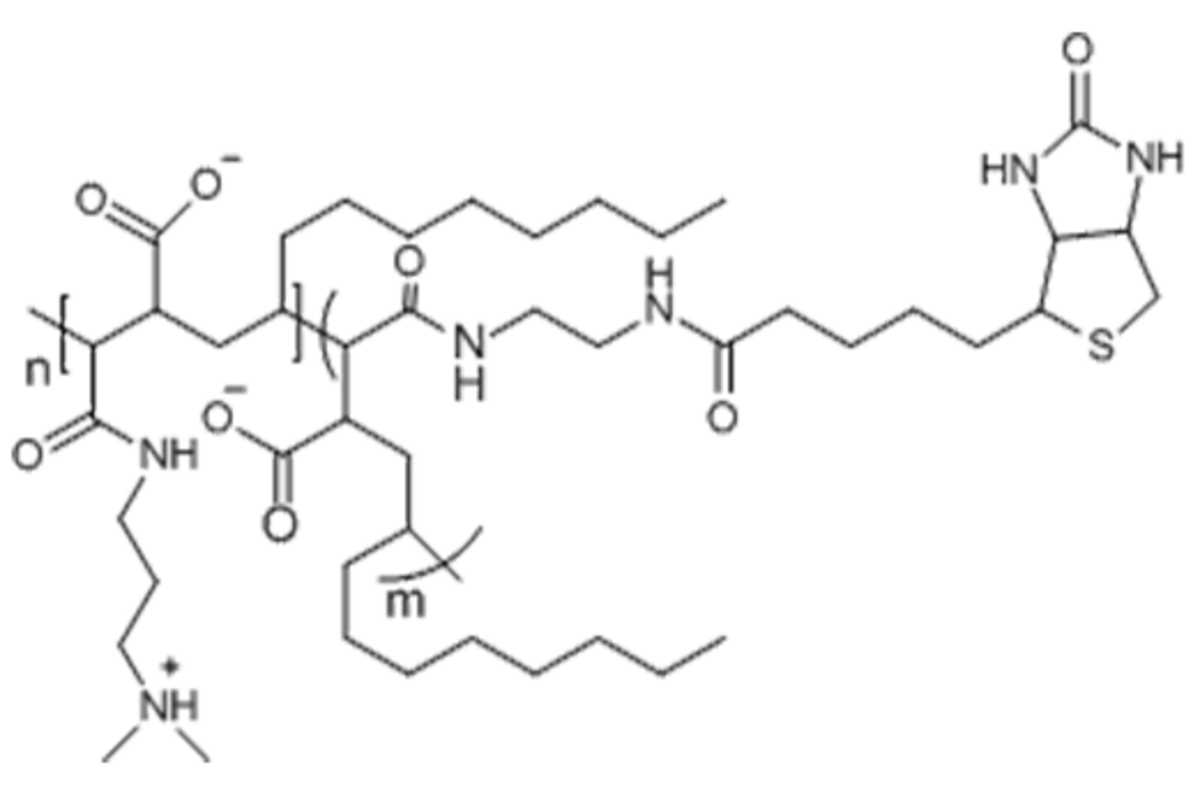
Name: BAM01 - Biotinylated A8-35
Formula: (C6.5H11.225O1.375N0.75Na0.325)~72
Cat No: BAM01
Pack Sizes: 50 mg, 100 mg, 500 mg
Name: Biotinylated PMAL-C8 - Poly (Maleic Anhydride-alt-1-Decene) substituted with 3-(Dimethylamino) Propylamine and Biotin ethylene diamine
Formula: (C 19H36O3N2)n.(C26H43O5N4S)m | n≈97-99% (avg. 52.4-53.5 units); m≈1-3% (avg. 0.5-1.6 units)
Cat No: BP5008
Pack Sizes: 50 mg, 100 mg, 250 mg
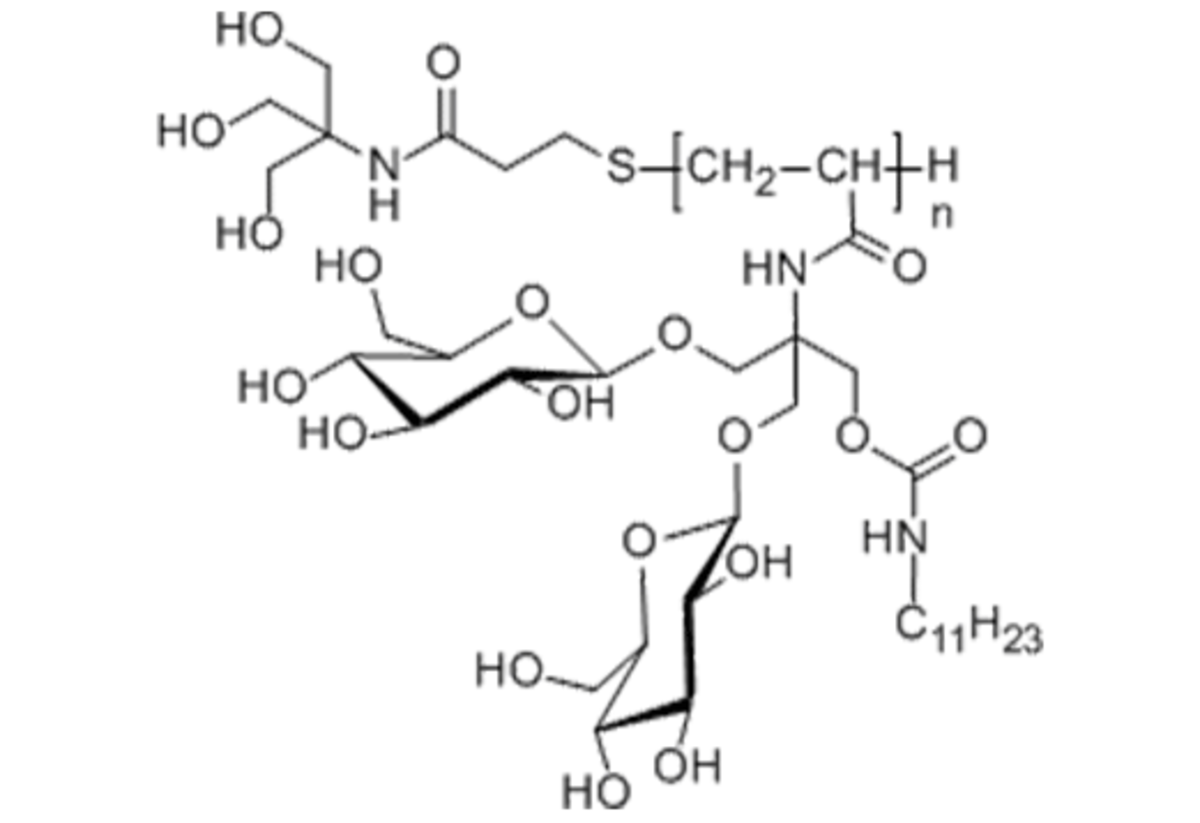
Name: NAPol - Non-Ionic Amphipol, Glycosylated - Non-Ionic Amphiphilic Homopolymers
Formula: C7H15O4SN (C31H54O15N2)n
Cat No: NAP
Pack Sizes: 10 mg, 100 mg, 250 mg, 500 mg
Removal of excess detergent
Whilst being invaluable tools for solubilising and stabilising MPs common detergents such as DDM or LMNG can also be problematic when trying to distinguish a protein particle from detergent by negative stain.
Removal of excess detergent typically requires additional, complicated steps such as dialysis and/or chromatography methods (e.g., ion exchange, SEC). The DetEx Mini Spin Columns are designed to rapidly and effectively remove excess detergent using weak ion exchangers. They are simple to use and require only a micro centrifuge.
| Cat-No. | Item | Size | Price (CHF) |
|---|---|---|---|
| PCL-MINI-4 | Proteus Detergent Cation Exchange Mini Spin Column Trial pack | 4 pieces | 199.00 |
| PCL-MINI-20 | Proteus Detergent Cation Exchange Mini Spin Column Kit | 20 pieces | 592.00 |
| PAL-MINI-4 | Proteus Detergent Anion Exchange Mini Spin Column Trial pack | 4 pieces | 207.00 |
| PAL-MINI-20 | Proteus Detergent Anion Exchange Mini Spin Column Kit | 20 pieces | 619.00 |
Conclusion
With CryoEM becoming ever more important in the drug discovery pathway the requirement to have a reliable supplier of high-quality reagents and tools is vital to achieving the best possible results. Anatrace have been, and will continue, leading the way in producing detergents and other molecules especially suited to CryoEM and working with membrane proteins providing you with total peace of mind.
References
- Sgro GG and Costa TRD (2018) Cryo-EM Grid Preparation of Membrane Protein Samples for Single Particle Analysis. Front. Mol. Biosci. 5:74
- Xu Y and Dang S (2022) Recent Technical Advances in Sample Preparation for Single-Particle Cryo-EM. Front. Mol. Biosci. 9:892459.
- Birch, J., Cheruvara, H., Gamage, N., Harrison, P.J., Lithgo, R., & Quigley, A. (2020). Changes in Membrane Protein Structural Biology. Biology, 9.
- Stetsenko A, Guskov A. An Overview of the Top Ten Detergents Used for Membrane Protein Crystallization. Crystals. 2017; 7(7):197.
- Denisov, I., Sligar, S. Nanodiscs for structural and functional studies of membrane proteins. Nat Struct Mol Biol 23, 481–486 (2016)
- Anatrace Newsletter, February 2020
Suppliers

Anatrace - For membrane protein studies
Anatrace develops and supplies high-purity detergents, lipids and reagents for membrane protein studies.

Protein Ark - protein sample preparation
Protein Ark offers a wide range of products for the purification and filtration of proteins, incl. chromatography, FPLC and spin columns, and affinity resins.