About Bispecific Antibodies
A bispecific antibody drug (BsMAb, BsAb) is a synthetic protein that can bind to two distinct antigen types or two distinct epitopes on the same antigen at the same time. BsAbs can be produced in a variety of structural forms and can be created to induce protein complex interaction, interfere with receptor signaling, inactivate signaling ligands, and activate immune cells. They have been investigated for medication delivery, Alzheimer’s illness, and cancer immunotherapy.
Two distinct epitopes are recognized by bispecific antibodies (BsAbs). Wide-ranging applications are made possible by this dual specificity, including rerouting T lymphocytes to tumor cells, simultaneously inhibiting two signaling pathways, dual targeting of several disease agents, and carrying payloads to selected areas.
Structure and Function
Monoclonal antibodies serve as the foundation for the creation of BsAbs. Early on in the creation of BsMabs, hinged cysteine in monoclonal antibodies was reduced and then reoxidized to form them. Currently, they are split into two groups depending on their structures: antibodies based on immunoglobulin G (IgG) and antibodies based on variable fragment (Fv).
According to their intended use, BsAbs are categorized into three groups:
(i) antibodies that target two distinct tumor antigens;
(ii) antibodies that target one tumor antigen and one phagocytic molecule; and
(iii) antibodies that target two immune-related molecules.
Learn more about Bispecific Antibody Drugs: https://www.krishgen.com/blog/details/bispecific-antibody-elisa-for-drug-discovery
The BiTE Mechanism
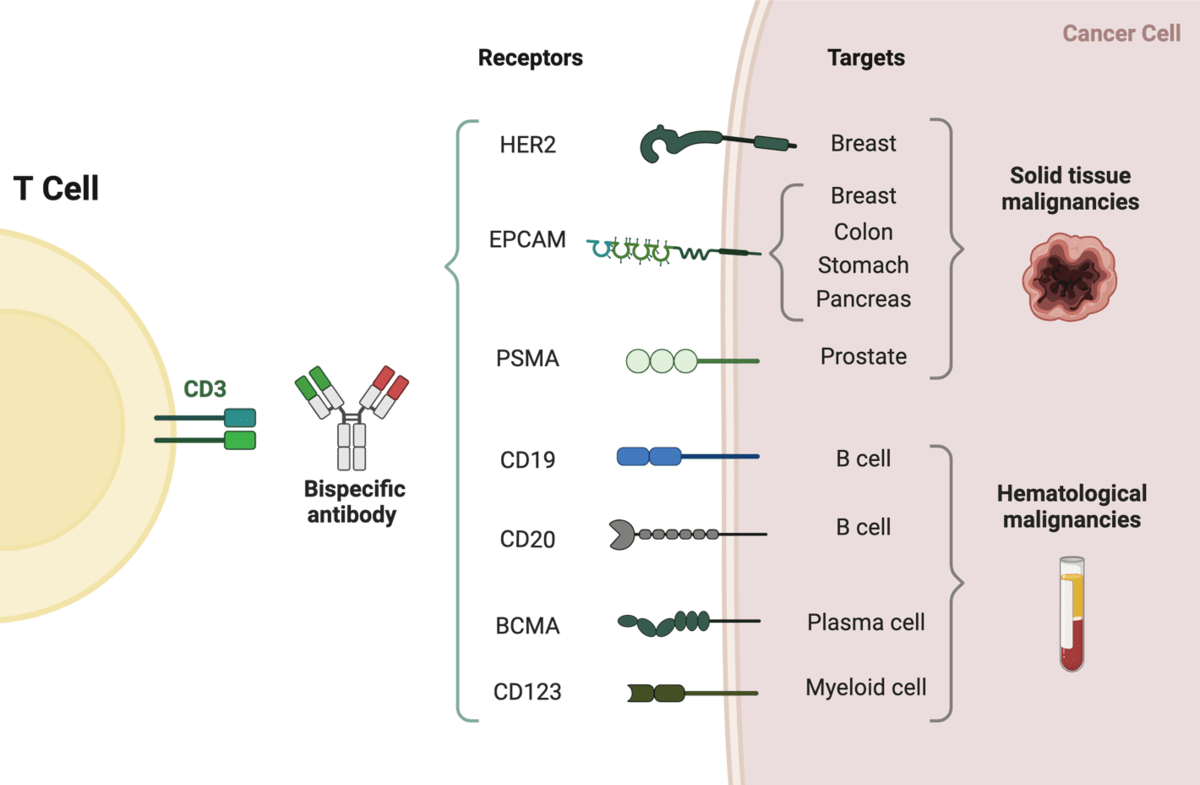
Bi-specific T-cell engagers (BiTEs) are a class of bispecific monoclonal antibodies that are investigated for use as anti-cancer drugs. They direct a host’s immune system, more specifically the T cells’ cytotoxic activity, against cancer cells. As one BiTE molecule often targets both a tumor antigen and a CD3 molecule concurrently, BiTEs fall under the second category of BsAbs.
The molecules of BiTE are antibody constructions with two binding domains: one that binds to tumor-expressed antigens (such BCMA, CD19, and DLL3), and another that binds to CD3 T-cells. The single-chain variable fragment (scFv) is an antibody fragment produced by fusing one variable region of the heavy chain (VH) and one variable region of the light chain (VL) artificially. Two scFv sections connected by a flexible peptide linker make up the antigen binding domains. The initial scFv connecting domain can be altered to attack any surface antigen, enabling re-treatment and off-the-shelf, rapid therapy against different cancers. The invariable component of the T-cell receptor complex, CD3, is always the focus of the second scFv binding domain.
Different from natural antibodies, BiTEs can redirect T cells to specific tumor antigens and activate T cells directly. When a BiTE molecule interacts with both a tumor cell and a cytotoxic T cell, the T cells begin to multiply, boosting the total number of effector cells and enhancing the effectiveness of BiTE therapy. Then, tumorigenic lysis is initiated. BiTE molecules can engage any T cell since this occurs without the necessity for co-stimulation or normal major histocompatibility complex procedures.
Activated T cells release granzymes such as perforin and others via immunological synapses. These lytic proteins can create pores in the membrane of cancer cells. Perforin and other proteinases are released into the cytoplasm by cancer cells and create endosomes as part of the membrane self-repair process. The release of granzymes from perforin-containing endosomes can cause pores to appear on the endosomal membrane, which lyses cancer cells.
Clinical Significance
BiTEs, in contrast to natural antibodies, can lead T cells to certain tumor antigens and activate them there. Because T- lymphocytes lack Fc receptors, natural antibodies cannot directly attract T cells. The CD3 molecule forms a non-covalent bond with the TCr and takes part in the transmission of antigen-specific signals that can cause T cells to become activated. High quantities of integrin-b1 and Interleukin-2 receptor alpha chain are expressed by activated T cells, and these molecules encourage T cell proliferation.
BiTE treatment is also a potential method for reactivating worn-out T cells brought on by repeated exposure to tumor antigens. There have been some observed characteristics of activation of T cells brought on by BiTEs. First, the tumor cell is crucial to the activation of T cells that the BiTE causes. Second, costimulatory signals like CD28 and interleukin (IL)-2 are not necessary for T cells to become activated. This characteristic is linked to memory T cells, which are crucial to the response to BiTEs.
Another theory is that TCrs can be assembled, and initial signals can be amplified by immunologic connections across T cells and tumor cells.
The first and only authorized BiTE therapy, blinatumomab, aims at the CD19 receptor on both healthy and cancerous B cells. It is an extremely powerful molecule with anticancer activity seen at limited exposures (10-100 mg/mL), and in the presence of it, T cells can carry out serial-target lysis, quickly binding and killing a large number of cells. Patients with Acute lymphocytic leukaemia and non-Hodgkin lymphoma have shown that blinatumomab is an effective treatment.
Succinctly, bsAbs have advantages over both monoclonal antibody drugs, as well as combination therapies. BsAb have displayed higher binding avidity to targets and have boosted cytotoxic effects. One of the main challenges of mAb drugs have been drug resistance, which reduces with bsAb drugs. Additionally, due to their unique structure and binding abilities, they are able to generate new functionalities that are not present in any combination of parent antibodies.
More about BiTE Antibody Drugs: Clinical Acceptance
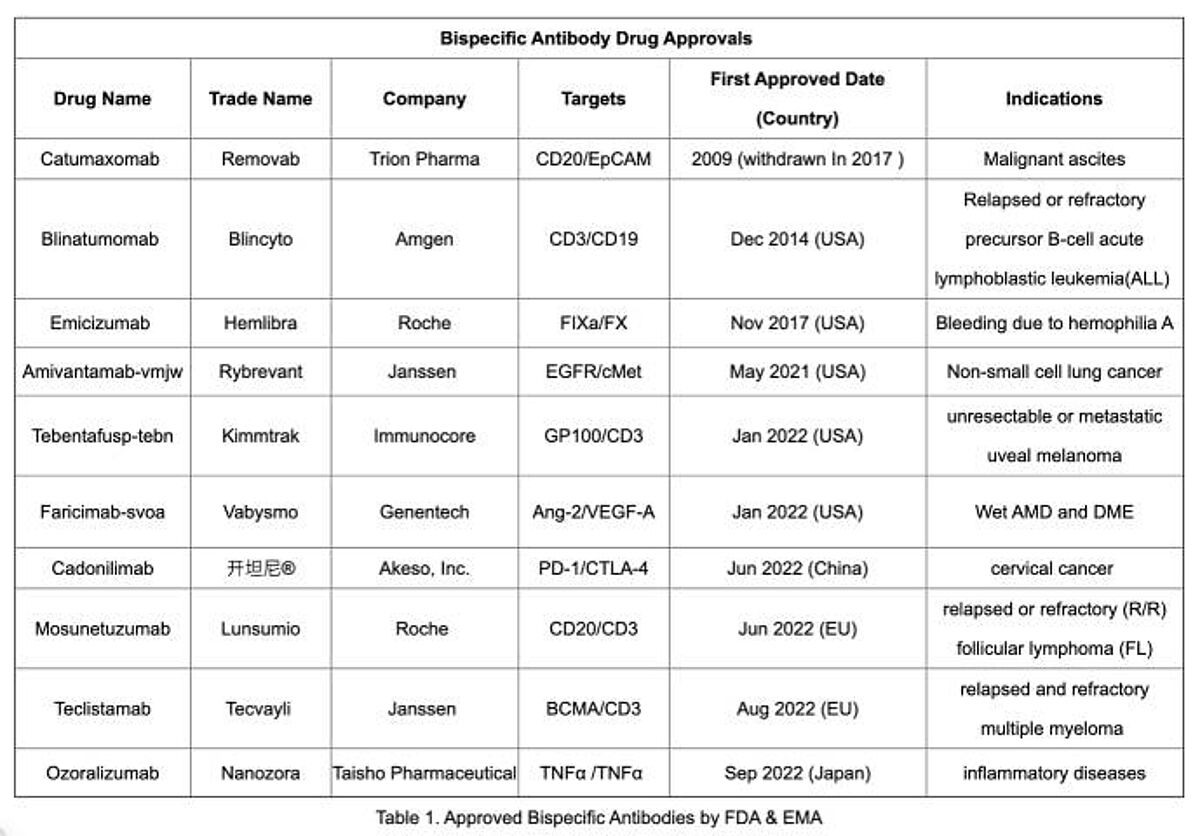
Currently, there are nine bispecific antibodies approved worldwide, among them, five bispecific antibodies approved by FDA, including blinatumomab, emicizumab, amivantamab, tebentafusp-tebn and faricimab-svoa.
Bispecific Antibody Drug Research
Bioanalytical assessment alongside development and optimisation of BiTE drugs is an imperative step. The appropriate pharmacological quantification in vitro may help to improve the therapeutic index of these immune agonists, allowing for the selection of the most favorable compounds during early drug discovery. Appropriate quantification is also critical for predicting a clinically relevant, safe, and pharmacologically active starting dose. This is a key challenge for CD3-bispecific therapy development.
Krishgen is currently the world’s only manufacturer developing commercial ELISA for the pharmacokinetic studies of bispecific antibody drugs. With 40+ drug targets available in a sensitive, highly specific ELISA format, Krishgen can support your bsAb research.
To ensure sensitivity and robustness, the method employed by all of Krishgen’s ELISA for bsAb is the sandwich assay technique. The proteins used for both coating and detection are the two that binding to the antibody. In the case of Teclistamab, for example, recombinant BCMA protein is pre-coated onto microwells, and the detection protein is an HRP-conjugated CD3 molecule. For Blinatumomab, the wells are pre-coated with CD-19, and once again, an HRP-conjugated CD3 acts as the detection.
Find an ELISA: https://krishgen.com/bispecific-antibody-drug-elisa/
Quick References
Einsele, H., Borghaei, H., Orlowski, R.Z., Subklewe, M., Roboz, G.J., Zugmaier, G., Kufer, P., Iskander, K. and Kantarjian, H.M. (2020), The BiTE (bispecific T-cell engager) platform: Development and future potential of a targeted immuno-oncology therapy across tumor types. Cancer, 126: 3192-3201. https://doi.org/10.1002/cncr.32909
Amgen: https://www.amgen.com/stories/2018/08/the-shape-of-drugs-to-come/bispecific-antibody
Tian, Z., Liu, M., Zhang, Y. et al. Bispecific T cell engagers: an emerging therapy for management of hematologic malignancies. J Hematol Oncol 14, 75 (2021). https://doi.org/10.1186/s13045-021-01084-4
Biochempeg: Approved Bispecific Antibody Drugs
Brennan M, Davison PF, Paulus H. Preparation of bispecific antibodies by chemical recombination of monoclonal immunoglobulin G1 fragments. Science. 1985;229(4708):81–3
Engelberts PJ, Hiemstra IH, de Jong B, Schuurhuis DH, Meesters J, Beltran Hernandez I, et al. DuoBody-CD3xCD20 induces potent T-cell-mediated killing of malignant B cells in preclinical models and provides opportunities for subcutaneous dosing. EBioMedicine. 2020;52:102625
Nagorsen D, Baeuerle PA. Immunomodulatory therapy of cancer with T cell-engaging BiTE antibody blinatumomab. Exp Cell Res. 2011;317(9):1255–60
Brischwein K, Parr L, Pflanz S, Volkland J, Lumsden J, Klinger M, et al. Strictly target cell-dependent activation of T cells by bispecific single-chain antibody constructs of the BiTE class. J Immunother. 2007;30(8):798–807
Supplier

Krishgen Biosystems
Krishgen Biosystems is an original manufacturer of therapeutic antibody ELISA kits, HCP detection assays, immunotherapy assay kits, antibodies, proteins, assays for drug development.
About Krishgen Biosystems Shop for Kirshgen Biosystems products
