By Stuart P. Atkinson, Ph.D., May 5, 2022 (first published by Active Motif)
Introduction
While the complex interconnections between our brain and intestine may provide us with some degree of intuition as our daily lives play out, gut feelings are not always welcome or helpful! Inflammatory conditions impacting the intestine can significantly impact the well-being of millions of people across the globe. Understanding the mechanisms that control intestinal health and applying these insights to develop novel therapeutics are just some of the ongoing research and opportunities to improve gut health.
Environmental Assault – Intestinal Epithelial Cells as Physical & Biochemical Barrier
As environmental factors play an outsized role in the development of inflammatory conditions (such as inflammatory bowel disease; Khor et al.), epigenetic mechanisms likely play a crucial role in their pathology (Jenke and Zilbauer). Researchers led by a duo of Frank Lyko (German Cancer Research Center, Heidelberg, Germany) and Yehudit Bergman (Hebrew University Medical School, Jerusalem, Israel) also understood that the gut microbiome held the potential to modify the epigenetic landscape of specific intestine-resident cells (Thaiss et al. and Gury-BenAri et al.), which would impact gene expression and intestinal cell function, key to maintaining intestinal homeostasis and avoiding disease development. Their recently published study explores how the gut microbiome impacts the epigenetic landscape of intestinal epithelial cells. These gut guardians provide a physical and biochemical barrier to segregate host tissue and the gut bacteria to maintain intestinal homeostasis (Petersin and Artis), under normal conditions and in response to the acute inflammation associated with inflammatory bowel disease. Excitingly, their findings suggest that the activities of the gut microbiome support the generation of a specific epigenetic profile at the regulatory elements of intestinal health-associated genes. The authors hypothesize that this mechanism may help to support a gene expression profile that supports intestinal homeostasis and may also protect against the consequence of gut inflammation.
Transcriptomics – With Germs and Without
Transcriptomic comparisons between intestinal epithelial cells isolated from both germ-free and conventionally raised male mice (C57BL/6) of six-to-seven weeks of age robustly indicated the presence of a gene expression profile driven by exposure to the gut microbiome. Downregulated genes in conventionally raised mice included those influencing extracellular matrix and metabolic processes, while upregulated genes mainly impacted cell proliferation. Overall, these initial findings indicated that the gut microbiome might significantly impact multiple aspects of intestinal epithelial cell biology.
DNA Methylation at Gene Regulatory Regions – Under the Influence of Microbes
Subsequent epigenetic analysis employing whole-genome bisulfite sequencing (WGBS) provided evidence for a global reduction in DNA methylation levels at gene-regulatory elements in intestinal epithelial cells isolated from conventionally raised mice compared to germ-free mice. Interestingly, gene ontology analysis of upregulated genes that underwent DNA methylation loss at regulatory regions in conventionally raised mice offered terms that included “mouse colitis,” “human inflammatory bowel disease,” and “human senescence.” With this knowledge in hand, the authors proposed that the exposure to gut bacteria promoted DNA demethylation at gene-regulatory regions to promote the expression of genes that protect the intestine and promote homeostasis through antibacterial and anti-inflammatory activities (e.g., IFITM3, NOS2, and PLA2G2A), which the authors termed “early sentinel” response genes.
Experimentally-Induced Bacterial Exposure – a Controlled System
The study next evaluated intestinal epithelial cells after the exposure of conventionally raised mice to dextran sodium sulfate, a synthetic sulfated polysaccharide that alters intestinal mucus layers, allowing bacterial infiltration to mimic the development of inflammatory bowel disease. Interestingly, these inflammatory conditions promoted DNA methylation loss at gene-regulatory regions and an increase in associated gene expression. Interestingly, the authors noted that the association of these inflammation-induced genes with colitis and colon cancer might provide a link that bridges epigenetics, inflammation, and cancer (Elinav et al.).
Notably, the authors also observed a heightened sensitivity of germ-free mice to experimentally induced inflammatory bowel disease and a significant reduction in DNA methylation-induced changes to gene expression (including the previously identified protective genes) compared to conventionally raised mice. These findings implied a role for the gut microbiome in stimulating the vast majority of inflammation-induced gene expression observed in conventionally raised mice. In confirmation of this hypothesis, fecal transplants from conventionally raised to germ-free mice prompted a significant reduction in DNA methylation at gene-regulatory elements and the transcriptional upregulation of associated genes in isolated intestinal epithelial cells, thereby confirming the critical role of the microbiome in epigenetic programming of intestinal cells.
Gene Enhancer Regions – What Can be Revealed with ATAC-Seq
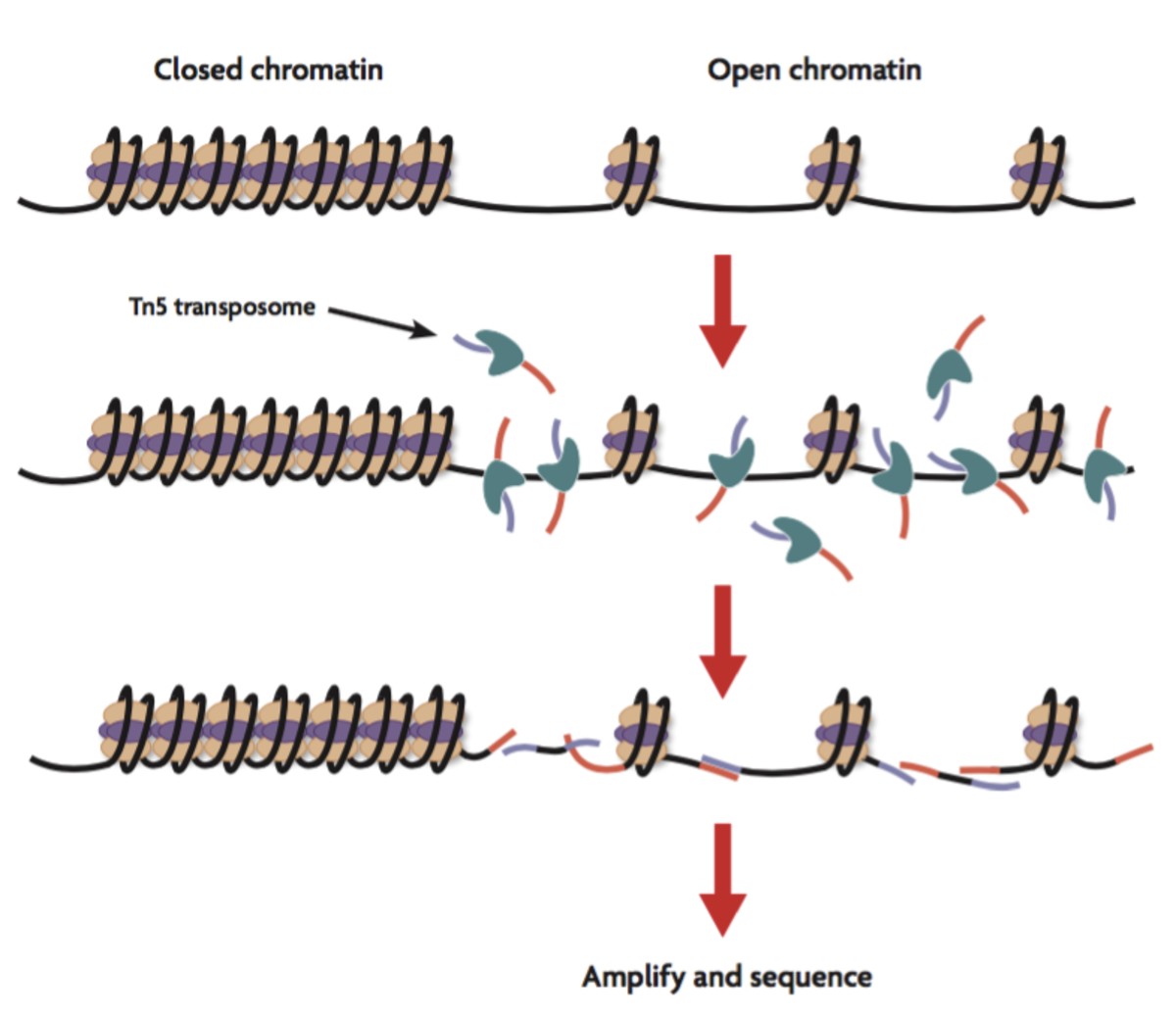
A deeper epigenetic analysis of the regulatory elements impacted by experimentally induced inflammatory bowel disease in conventionally raised mice suggested that the associated acute inflammation-induced profound epigenetic changes at putative gene enhancer regions (marked by the monomethylated H3K4 and acetylated H3K27 histone modifications); furthermore, the alterations to the epigenetic landscape occurred concurrently with the altered activity of inflammatory transcriptional networks controlled by the NF-κB and AP1 transcription factors. Additional evaluation of the epigenetic landscape via the application of genome-wide assay for transposase-accessible chromatin using sequencing (ATAC-Seq) also showed that upregulated gene expression in intestinal epithelial cells from inflammatory bowel disease model mice, associated with increased chromatin accessibility, which agrees well with the associated loss of DNA methylation.
TET Enzymes – the Microbiome Effect
Finally, the authors addressed the molecular mechanics behind gut microbiome-induced DNA methylation alterations and highlighted the elevated expression of the TET2 and TET3 methylcytosine dioxygenases, which play well-understood roles in the DNA demethylation process, in conventionally raised mice compared to germ-free mice. Their study discovered that the loss of TET2 and TET3 expression in conventionally raised mice inhibited the previously observed inflammation-induced loss of DNA methylation at regulatory regions, promoting greater susceptibility to inflammatory bowel disease symptoms. Their work showed that TET2 and TET3-deficient mice displayed a reduced response to inflammatory challenges.
Summary
Overall, these results suggest that the presence of the gut microbiome supports the formation of a specific epigenetic landscape at gene regulatory regions in intestinal epithelial cells, which encourages the expression of genes that support normal gut health and may protect against the pathological consequences of inflammatory conditions such as inflammatory bowel disease.
For more details on how your gut microbiome helps protect you from unwanted gut feelings, see Nature Microbiology February 2020.
More Information
About the author – Stuart P. Atkinson, Ph.D.
Stuart was born and grew up in the idyllic town of Lanark (Scotland). He later studied biochemistry at the University of Strathclyde in Glasgow (Scotland) before gaining his Ph.D. in medical oncology; his thesis described the epigenetic regulation of the telomerase gene promoters in cancer cells. Following Post-doctoral stays in Newcastle (England) and Valencia (Spain) where his varied research aims included the exploration of epigenetics in embryonic and induced pluripotent stem cells, Stuart moved into project management and scientific writing/editing where his current interests include polymer chemistry, cancer research, regenerative medicine, and epigenetics. While not glued to his laptop, Stuart enjoys exploring the Spanish mountains and coastlines (and everywhere in between) and the food and drink that it provides!