Recombinant Prokaryotic Lectins: Novel Tools for Analyzing and Purifying Glycoproteins
Lectins are widely used for analyzing and purifying glycosylated biomolecules, including therapeutic antibodies and other biologic drugs. Recombinant prokaryotic lectins (RPLs) offer several advantages over traditional plant-based lectins and are seeing increased uptake for multiple applications.
What are lectins?
Lectins are a class of proteins which bind glycans, the chain-like structures of carbohydrate molecules (monosaccharides) produced by almost all living organisms. Their main purpose is to provide a means of defense, typically through lectin-mediated agglutination mechanisms (e.g., hemagglutination). However, lectins also have many other important functions, including sugar transport and storage, regulation of cell adhesion, and nitrogen fixation, depending on the species in question. Lectin concentrations are highest in raw legumes such as beans, lentils, and peas, as well as in whole grains like wheat, which have consequently seen widespread use as native lectin sources. Well known examples of plant lectins include wheat germ agglutinin (WGA), Erythrina cristagalli lectin (ECL), peanut agglutinin (PNA), and the highly potent toxin, ricin.
How are lectins useful for scientific research?
One of the main research applications of lectins is as tools for analyzing glycosylation, a post-translational modification that can influence protein structure and function. This usually involves using high performance liquid chromatography (HPLC) or mass spectrometry (MS) based methods, which provide highly detailed information about the sample being interrogated but are limited in terms of throughput. Such techniques are essential to the development and manufacture of biologic drugs since the glycosylation profile represents a critical quality attribute (CQA) for assuring drug safety and efficacy.
Lectins are also commonly used for a technique known as enzyme-linked lectin assay (ELLA), which is similar to ELISA and can be configured in several different ways. During a direct ELLA, the microplate wells are coated with the sample, then an enzyme-conjugated lectin is added to enable detection of bound glycoforms. Alternatively, a sandwich ELLA uses one lectin for capturing free glycoforms in solution and another, enzyme-conjugated lectin for detection. It is also possible to employ a hybrid approach, combining an antibody for target capture with an enzyme-conjugated lectin for detection. The chosen method will largely be dictated by the aim of the study.
Another familiar application of lectins is as affinity ligands for protein purification, via their immobilization on columns for chromatography-based extraction of glycosylated targets from solution.
What are the advantages of recombinant prokaryotic lectins?
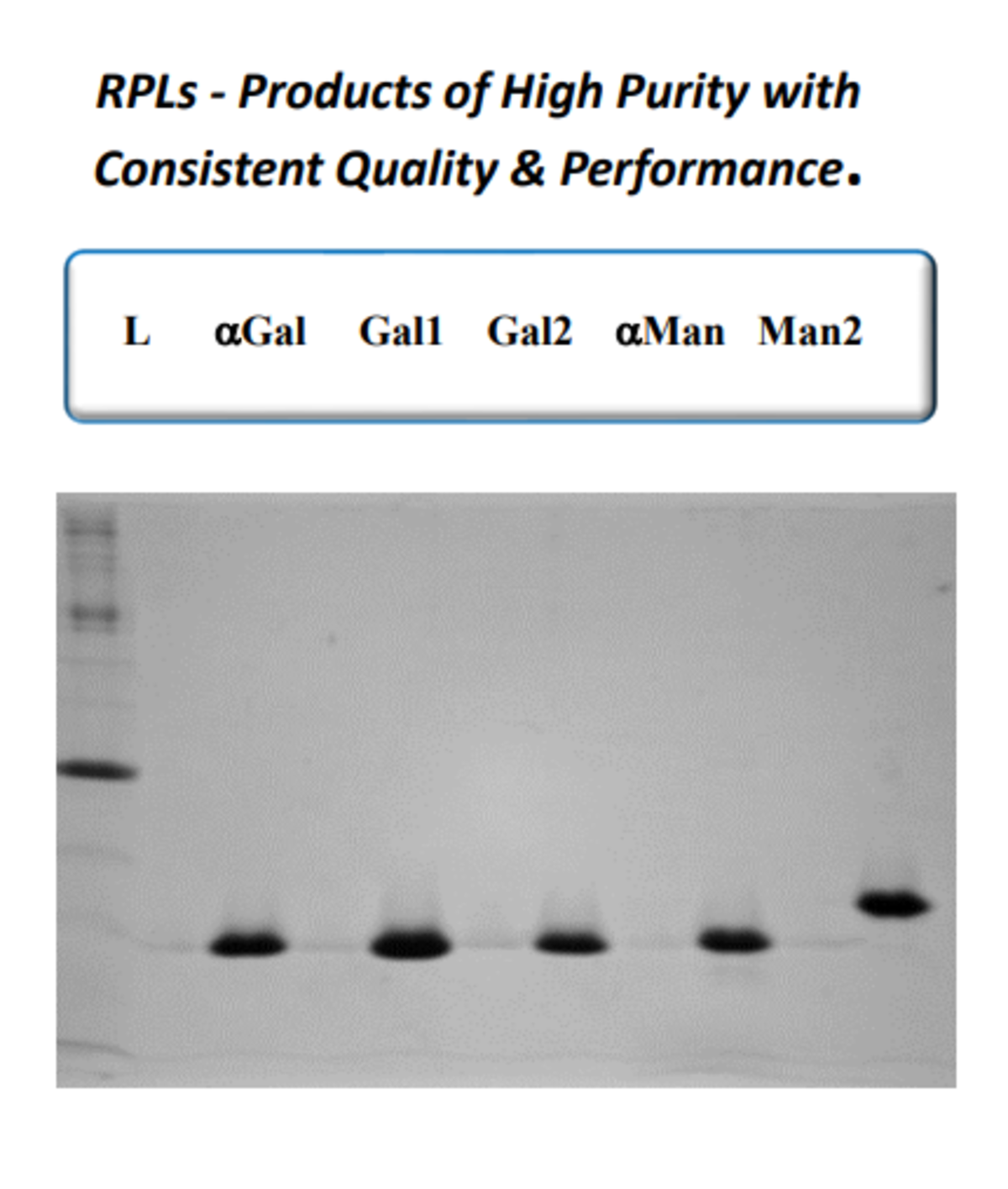
While native, plant-derived lectins have been used by researchers for decades, their low specificity, high batch-to-batch variation, and limited production scale have driven demand for alternatives. One company addressing this need is GlycoSeLect, which has developed a range of recombinant prokaryotic lectins by identifying targets of interest for expression in E. coli.
A major advantage of RPLs is that they each bind a single glycan epitope, giving them improved specificity over plant-derived lectins. Moreover, because RPLs have a known gene sequence, glycan binding can be further enhanced via targeted mutagenesis. Other benefits of RPLs include consistent batch-to-batch performance, high purity, and scalable production. In addition, RPLs can be engineered to express tags for increased workflow flexibility. For example, biotinylated RPLs are readily immobilized on surfaces by exploiting the high affinity biotin-streptavidin interaction, while His-tagged RPLs provide opportunities to incorporate anti-tag antibodies into experimental protocols.
How are RPLs being used?
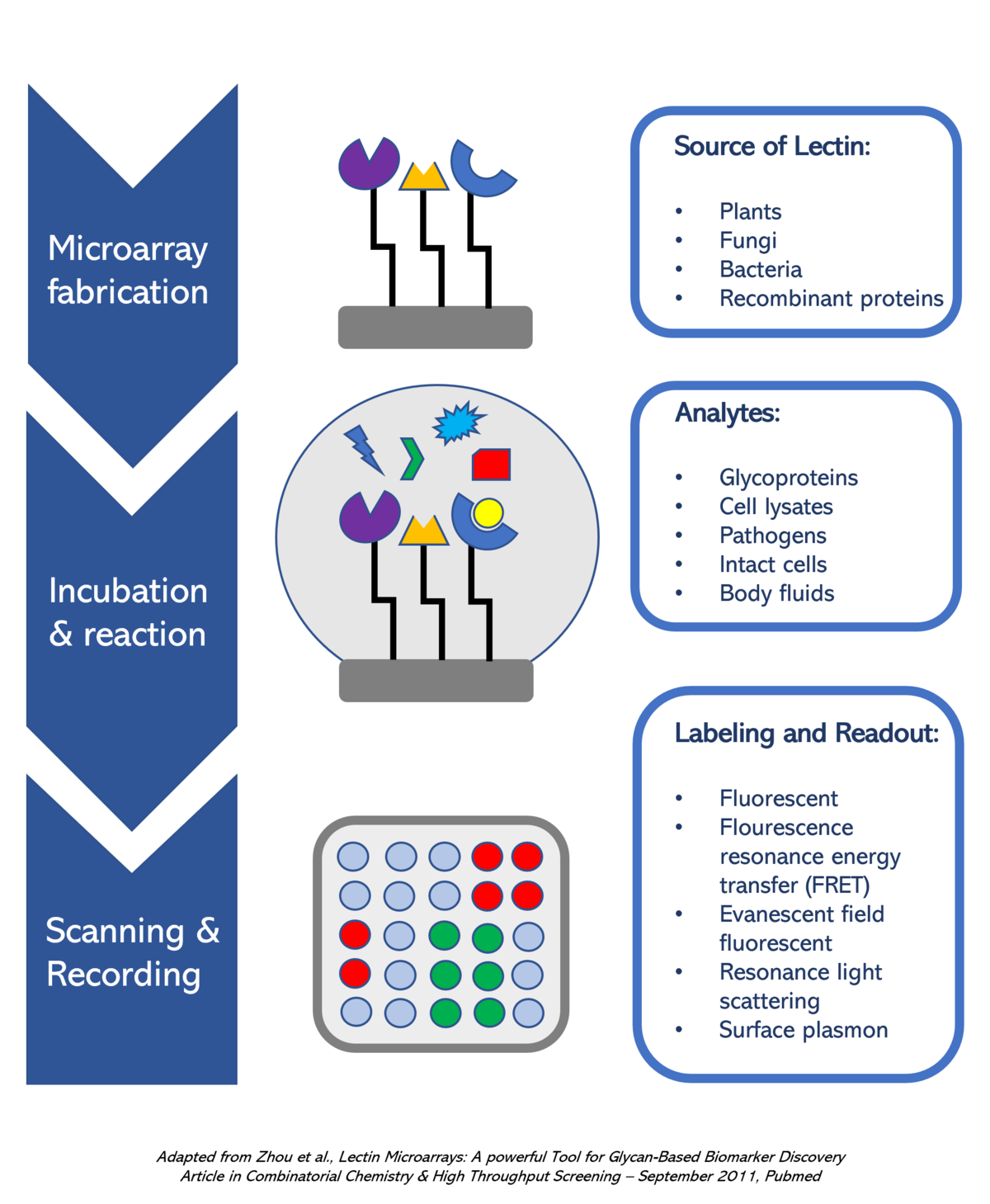
One way in which RPLs are being used is within the development of microarrays. Lectin microarrays are a high throughput, easy-to-handle, and highly efficient technology for determining the glycan composition from a complex mixture.By immobilizing a selection of different RPLs on a solid surface such as a nitrocellulose membrane or a functionalized glass slide, it is possible to simultaneously detect multiple glycoforms in solution.
The figure on the right shows the procedure for a typical lectin microarray experiment. (a) Lectins are delivered and immobilized on the microarray surfaces through a variety of surface chemistries. (b) Lectins could be obtained from a wide range of sources. (c) The glycosylation status of a wide range of biological samples are analyzed on lectin microarrays. (d) The results are recorded by either a standard fluorescence microarray scanner or other labeling and recording methodologies.
RPLs have also been immobilized on biosensors and used for biolayer interferometry (BLI) – a label-free technology for real-time monitoring of biomolecular interactions. Recently, researchers at Teesside University used BLI to compare traditional plant-derived lectins with RPLs having affinity for the same glycoforms. Their data, published in Analytica Biochimica Acta2, showed that while both sets of biomolecules had similar specificities for their targets, the RPLs exhibited higher binding activities and could be engineered to recognize different glycans.
RPLs also have potential utility as biomarkers for diagnosing specific health conditions, monitoring disease progression, and even in helping guide the development of personalized treatments.
LubioScience represents some of the most trusted brands in research and offers a growing range of RPLs from GlycoSeLect.
To discuss how we can support your glycoanalysis or glycopurification goals, contact us today.
Products by GlycoSeLect
| Product Name (RPL) | Specificity | Plant Lectin Equivalent |
|---|---|---|
| RPL-αGal | Terminal α-linked Galactose & N-Acetylgalactosamine (GalNAc) | - |
| RPL-Gal1 | Terminal β-linked Galactose & N-Acetyllactosamine (LacNAc) | ECL |
| RPL-Gal2 | Terminal α-linked Galactose > N-Acetylgalactosamine (GalNAc) | - |
| RPL-Gal3 | Terminal α-linked Galactose | - |
| RPL-Gal4 | Terminal β-linked Galactose, N-Acetyllactosamine (LacNAc) & Lewis x (Lex) | ECL |
| RPL-αMan | Fucose/Mannose: Lewis a (Lea), Lewis x (Lex) & terminal α-mannose | GNL |
| RPL-Man2 | Terminal α -mannose | GNL, NPL |
| RPL-Sia1 | Terminal α2-3-linked Sialic Acid (Neu5Ac) – on both N-linked and O-Linked Glycans | Mal II |
| RPL-Sia2 | Terminal α2-3-linked Sialic Acid (Neu5Ac) on O-Linked Glycans | PNA Lectin |
| RPL-Sia3 | Terminal α-linked Neu5Ac | WGA LEctin |
| RPL-Fuc1 | α-linked Fucose | - |
Recommended Antibodies by Proteintech
| Cat-No. | Item | Size | Price (CHF) |
|---|---|---|---|
| 66005-1-IG-150UL | 6*His, His-Tag Monoclonal antibody | 150 ul | 250.00 |
| 60003-2-IG-150UL | MYC tag Monoclonal antibody | 150 ul | 250.00 |
| 66008-4-IG-150UL | DYKDDDDK tag Monoclonal antibody (Binds to FLAG tag epitope) | 150 ul | 250.00 |
| 66003-1-IG-150UL | MBP tag Monoclonal antibody | 150 ul | 250.00 |
Suppliers

GlycoSelect
GlycoSeLect Ltd specialises in the development and production of Recombinant Prokaryotic Lectins (RPLs) for the Biopharmaceutical, Research & Diagnostics, Cosmetics, and Food industry.
About GlycoSelect Shop for GlycoSelect products

Proteintech - The benchmark in antibodies
Primary antibodies, nanobodies, cytokines & growth factors - all made in-house. Strict validation by western blot, ELISA and siRNA testing.
References
- Zhou SM, Cheng L, Guo SJ, Zhu H, Tao SC. Lectin microarrays: a powerful tool for glycan-based biomarker discovery. Comb Chem High Throughput Screen. 2011 Sep;14(8):711-9. doi: 10.2174/138620711796504398. PMID: 21933110.https://pubmed.ncbi.nlm.nih.gov/21933110/
- Fernandez-Poza S, Padros A, Thompson R, Butler L, Islam M, Mosely JA, Scrivens JH, F Rehman M, Akram MS. Tailor-made recombinant prokaryotic lectins for characterisation of glycoproteins. Anal Chim Acta. 2021 Apr 22;1155:338352. doi: 10.1016/j.aca.2021.338352. Epub 2021 Feb 27. PMID: 33766322. https://pubmed.ncbi.nlm.nih.gov/33766322/