The ongoing COVID-19 pandemic has been declared a public health emergency of international concern by the WHO.
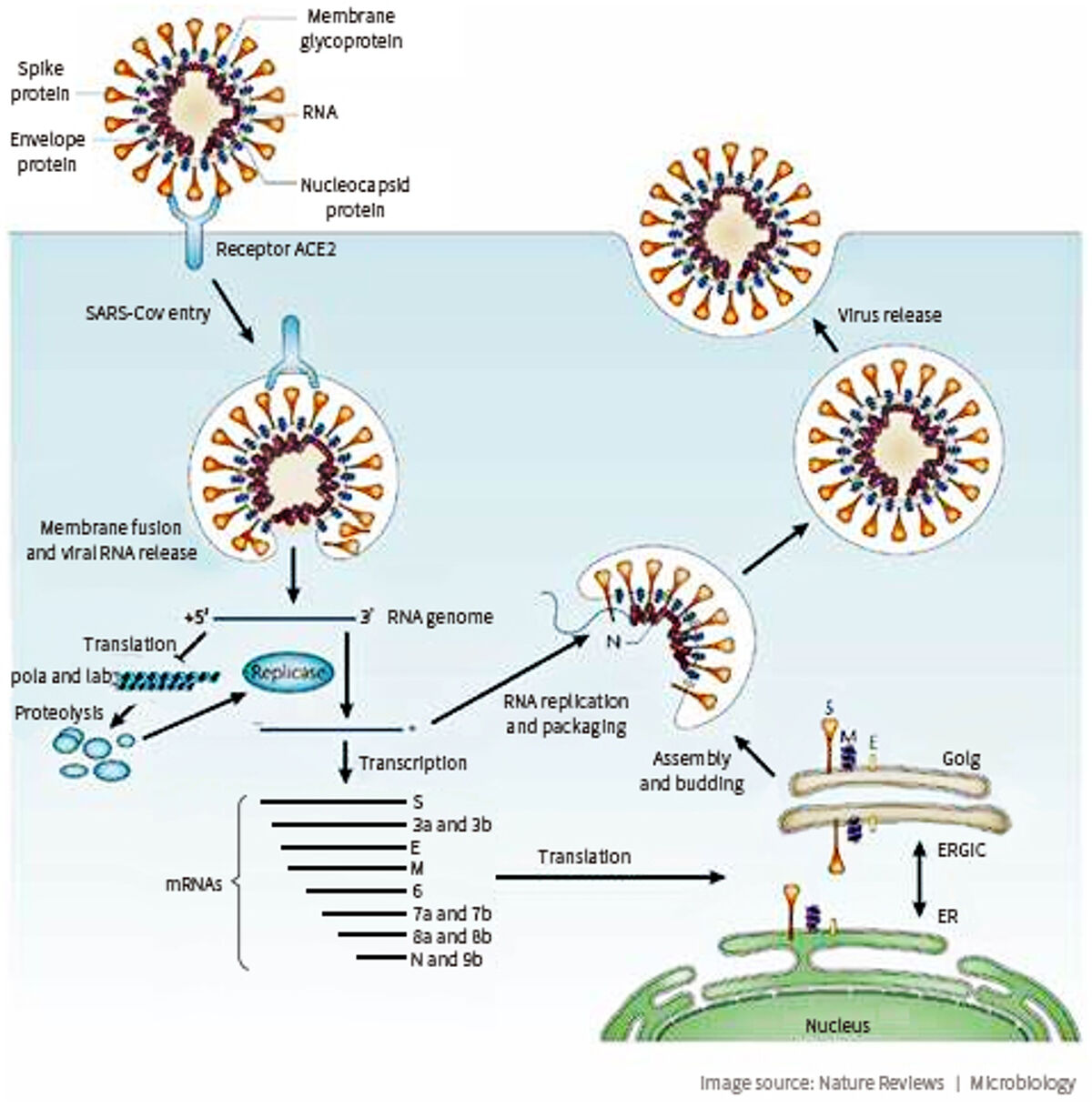
The causative SARS-CoV-2 virus attaches to cells by binding its outer “Spike” proteins to a receptor protein called ACE2 (angiotensin-converting enzyme 2) that is highly expressed on respiratory cells. ACE2 also acts as the receptor for the human respiratory coronavirus NL63 and the SARS-coronavirus (SARS-CoV). Studies have shown that the binding affinity of the virus Spike protein for ACE2 correlates with higher transmissibility of the coronavirus and contagiousness of the disease. Angiotensin receptor blockers have been proposed as tentative SARS-CoV-2 therapeutics. Interestingly, ACE2 activity may also have a protective effect against virus-induced lung injury by increasing the production of the vasodilator angiotensin.
What is ACE2?
Human ACE2 - or angiotensin-converting enzyme 2 - is a type I integral membrane protein. It contains 805 amino acids, with an N-terminal signal peptide, followed by an extracellular domain, a transmembrane domain and a small cytoplasmic domain. The active cartboxypeptide domain is exposed on the surface and is cleaved from the transmembrane domain by an enzyme known as a sheddase. The resulting soluble protein is released into the blood stream and ultimately excreted into urine.
ACE2 is expressed in spleen, liver, retina, placenta, brain tissue, heart, arteries, veins, endothelial tissue, macrophages, gastrointestinal system, lung alveolar epithelial cells and other tissue cells. It has been identified as an entry point for the SARS-CoV-2 coronavirus and therefore has become a hot target for research into strategies to prevent host infection and to limit the spread of COVID-19.
How does SARS-CoV-2 enter host cells?
The first step of viral infection is virus binding to a functional receptor on the surface of host cells. For SARS-CoV-2, that receptor is ACE2. ACE2 is expressed in alveolar epithelial cells and enterocytes of the small intestine, both of which are primary target cells for coronavirus infection.
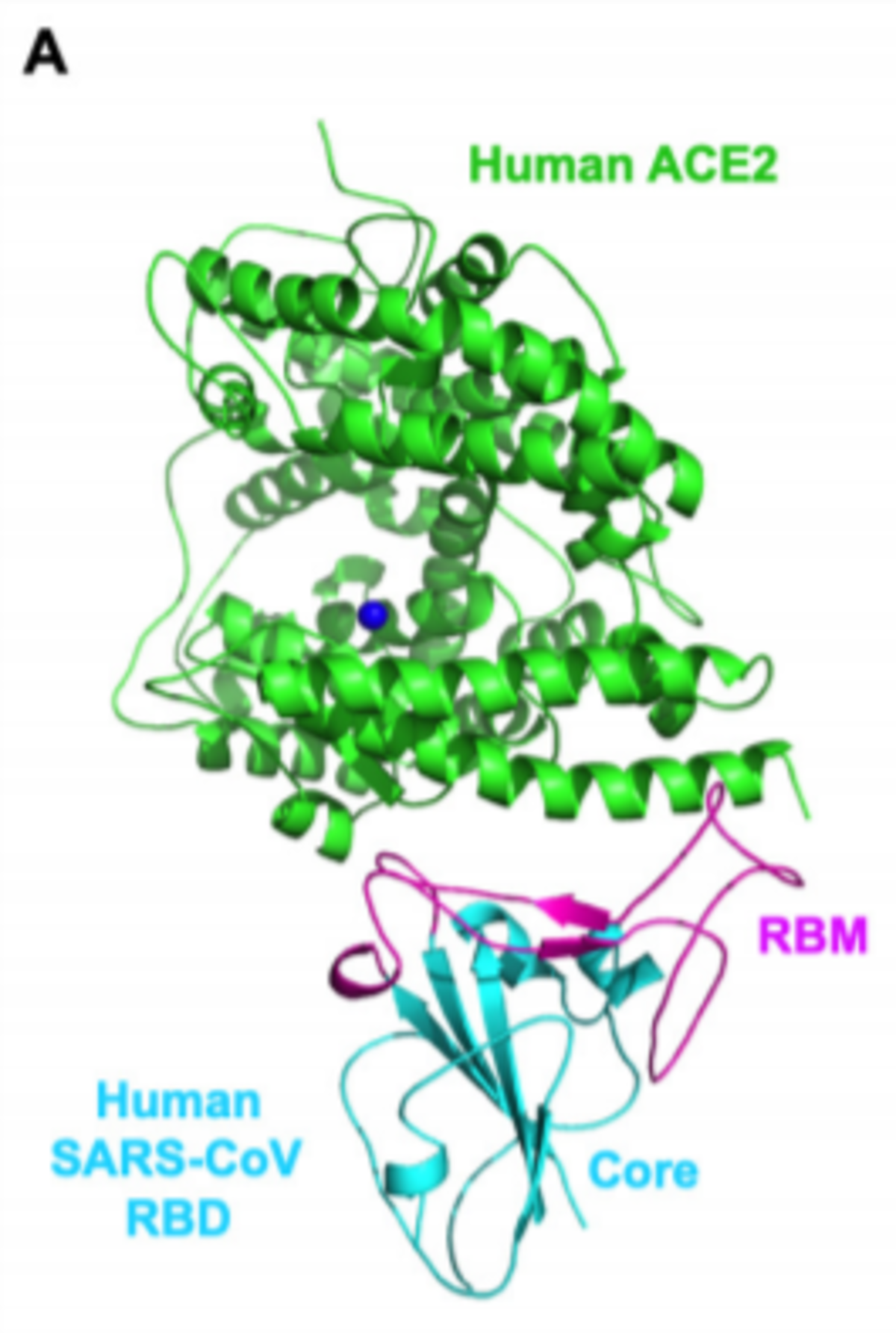
The spike protein (S-protein) of the coronavirus binds to the ACE2 receptor on the host cell, with the affinity of the receptor binding (RBD) domain of the S-protein for ACE2 determining the susceptibility of the host. On a molecular level, the viral S-protein binds to a claw-like structure of ACE2 and this interaction induces fusion between the viral and host cell membranes. Thus, small molecules or antibodies that can block the binding of S-Protein and ACE2 may be a promising approach to combat viral docking and fusion.
Strategies to fight SARS-CoV-2 host cell entry
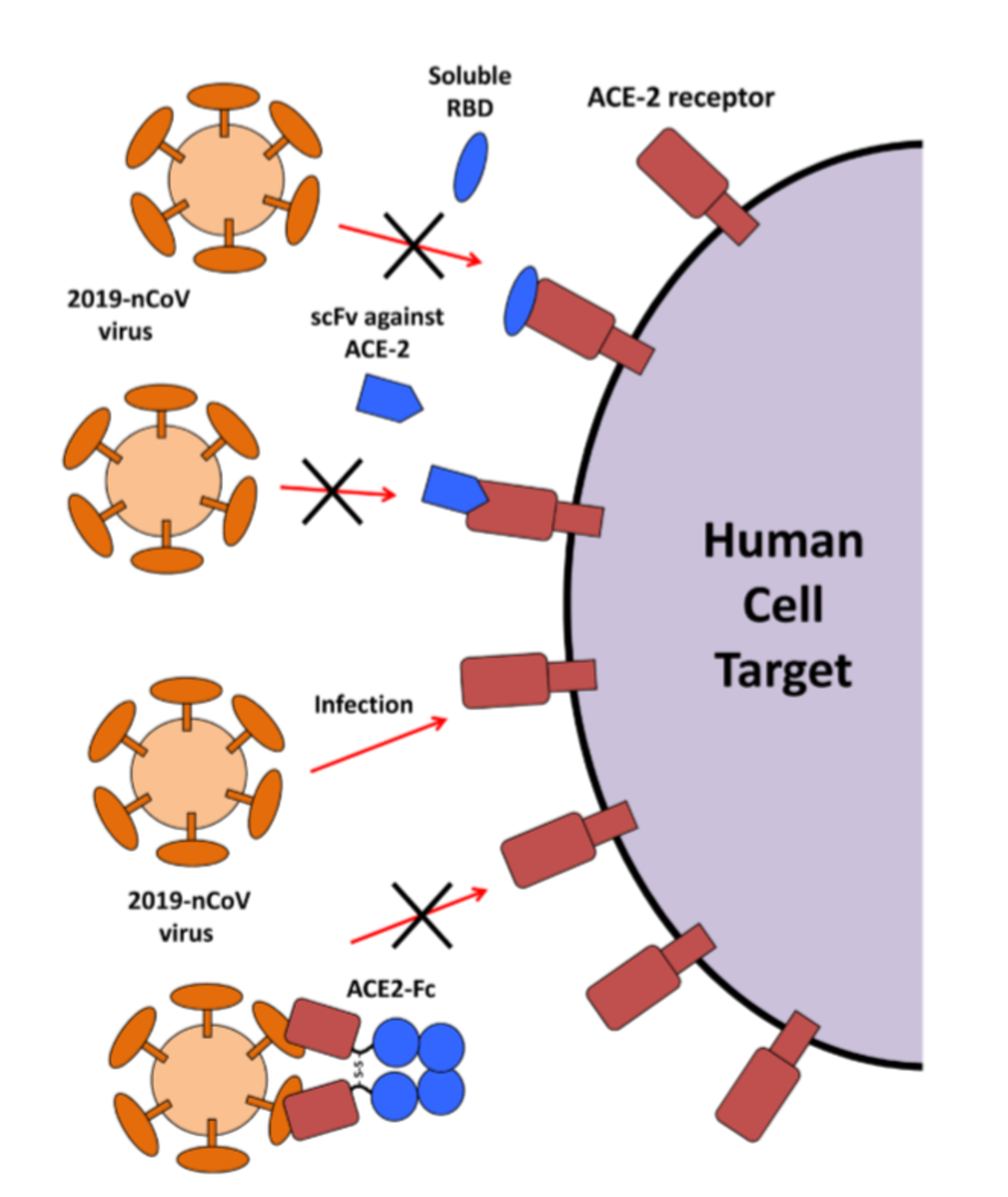
Based on the key step of S-protein binding to ACE2, several approaches have been proposed to prevent SARS-CoV-2 from infecting host cells:
- The RBD domain expressed on its own may act as a blocking agent to prevent the S-Protein-ACE2 interaction
- Similarly, antibodies scFv's blocking ACE2 may prevent viral attachment and fusion
- Use of the ACE2 extracellular domain as a soluble bait to sequester SARS-CoV-2
While certainly promising, it is important to keep in mind that these approaches are still in a proof-of-concept stage and significant research is needed to advance such concepts into a pre-clinical or even clinical stage.
Image credit
Figure 1 and Figure 2: Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARSdoi.org/10.1128/JVI.00127-20.
Figure 3: Therapeutic Strategies in an Outbreak Scenario to Treat the Novel Coronavirus Originating in Wuhan, China doi.org/10.12688/f1000research.22211.2