What's in the Tap Water
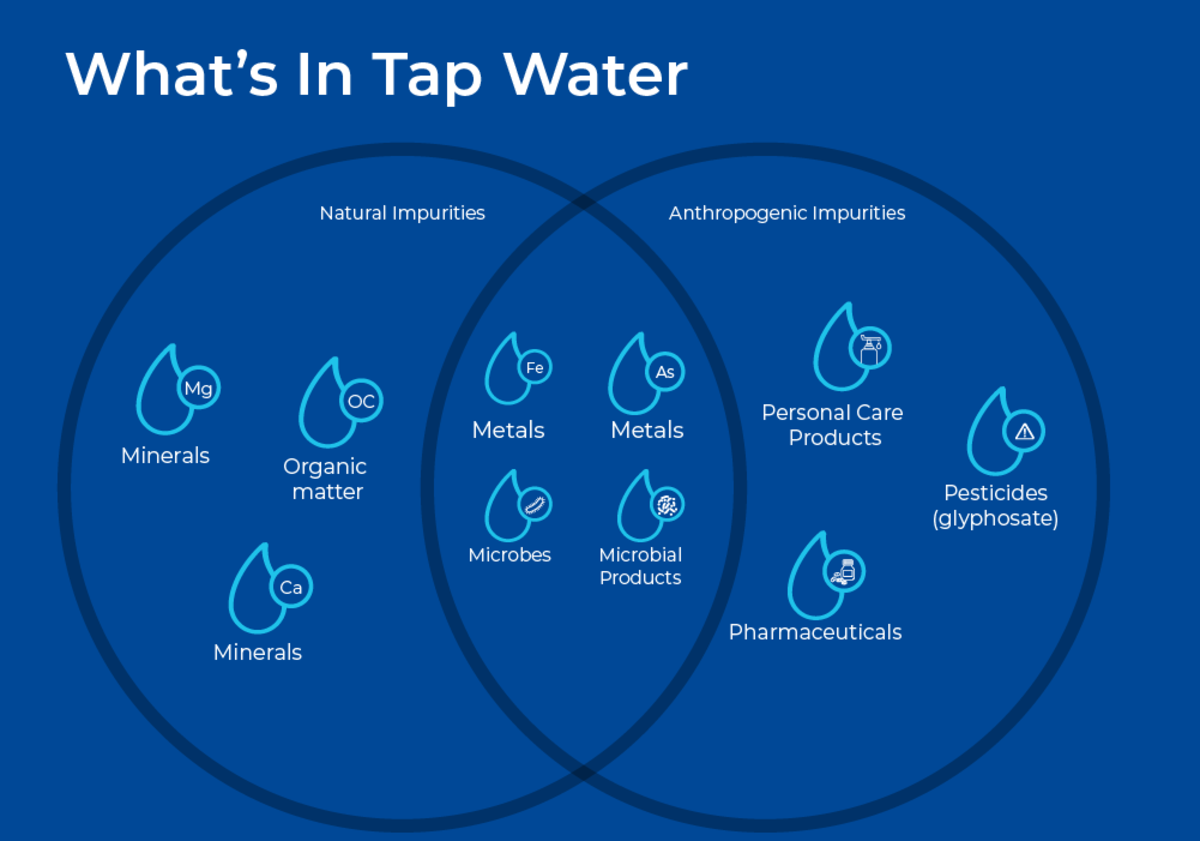
Although water that flows into most laboratories is safe for human consumption, it needs to be further purified for sensitive laboratory use. Water provided from most municipal water treatment systems still contains a wide range of natural minerals (eg. calcium, magnesium, sulphate etc.), dissolved organic matter, and toxic contaminants. Anthropogenic contamination of source water can lead to tap water that contains heavy metals (eg. lead, arsenic), inorganic chemicals (e.g. nitrates, and nitrites), organic chemicals (e.g., pesticides, industrial solvents), pharmaceuticals, and personal care products. Recent research also shows that water supplies all over the world, in both industrialized and rural settings, are contaminated with microplastics, with US tap water averaging 9.24 particles/L1. Moreover, the water treatment process itself can also produce contaminants such as chloroform, which can result as a disinfection by-product.
In addition to natural and industrialized chemicals, municipal water also contains microbial contamination. Water treatment systems do not completely remove all microbes, and trace amounts of different bacteria, fungi, or viruses can remain. Furthermore, even if microbial cells have been destroyed, their cell contents are released into the water upon lysis. Contaminants of microbial origin can include enzymes such as nucleases as well as endotoxins.

What are Nucleases
Nucleases are enzymes that degrade nucleic acids such as DNA and RNA by cleaving the phosphodiester bonds between nucleotides of nucleic acids. Nucleases belong to the hydrolase class of enzymes and are usually specific in action. Ribonucleases only act on ribonucleic acids (RNA), and deoxyribonucleases act only upon deoxyribonucleic acids (DNA). They can be further classified based on their target molecules and mode of action. Exonucleases digest nucleic acids from the ends, while endonucleases act on regions in the middle of target molecules
In living organisms these enzymes are important, as they are involved in the DNA repair process, proofreading during replication, and the removal of Okazaki fragment RNA primers from replication, among other processes. With respect to human health, mutations in these enzymes can have deleterious consequences, such as genetic instability or immunodeficiency. In a laboratory setting, the properties of these enzymes have been harnessed by researchers to perform genetic engineering such as CRISPR-Cas gene editing.
However, in most laboratory settings the presence of background nucleases is undesirable. These enzymes are extremely active, and a small amount of nucleases can produce a lot of nucleic acid degradation. It is therefore particularly important to keep all laboratory reagents and consumables nuclease-free.
What are Endotoxins
Endotoxins are a component of the outer membrane of Gram-negative bacteria. Gram-negative bacteria differ from most other cells as they have two cellular membranes. An inner membrane surrounds the cytoplasm, as in most cells, however, they also have an outer membrane that protects the cell from the external environment. This outer membrane contains a complex molecule called lipopolysaccharides (LPS). If introduced into higher organisms, LPS causes a wide range of toxic activities when present, even in small amounts (picogrammes). LPS is also considered a pyrogen, or a fever-causing agent. The term pyrogen stems from “pyro-” of the greek root “fire”, and “gen” from the greek “-genes”, meaning born of or produced by. Endotoxins are naturally found in the environment (water, air) populated by Gram-negative bacteria.
The Impact of Water Quality on Biological Research
Water that is free of chemical impurities, microorganisms, endotoxins, and enzymes such as nucleases and proteases is of utmost importance to ensure high quality research results.
- PCR: Contaminated water can introduce foreign DNA and/or nucleases during the amplification process, leading to inaccurate results. In addition, chemical impurities can inhibit the enzymatic reactions. Ultrapure nuclease-free water ensures that the DNA template remains pure from start to finish.
- RNA: For RNA isolation, cDNA synthesis, or RNA sequencing, it is crucial to maintain RNA integrity. Using RNase-free water when working with RNA prevents degradation and preserves the quality of the samples.
- Sequencing:Next-generation sequencing demands high-quality DNA templates. Nuclease-free water guarantees that the DNA sequences remain intact, reducing the risk of sequencing errors.
- Molecular Cloning: In the construction of recombinant DNA molecules, a wide variety of impurities can negatively impact the process. Ultrapure nuclease-free water ensures the integrity of the DNA fragments and efficiency of the cloning.
- Cell Culture: Ultrapure nuclease-free water is also vital for the preparation of cell culture media and buffers. Heavy metals such as lead, mercury, nickel, zinc, chromium, and cadmium are toxic to various cells. Endotoxins can inhibit cell growth and nucleases can compromise the integrity of cell lines and experimental results.

Water Quality Standards
Different regulatory agencies, such as ASTM International, define the purity of water based on measurable characteristics. Conductivity (measured in µS/cm), is an electrical measurement based on the flow of charged ions in the water. The conductivity indicates the concentration of dissolved ions in the water, and resistivity is the inverse of this. Type I water (ultrapure) has the lowest conductivity (highest resistivity), and Type IV water (semi-pure) has the highest conductivity (lowest resistivity). As there are also organic impurities that are not charged, Type I water is further defined by its total organic compounds (TOC). In addition to these 4 water types based on their chemical purity, there are three grade specifications based on contaminants of microbiological origin. Biological research laboratories have necessitated further classification based on the presence of specific enzymes such as nucleases and proteases.
Water Purification Methods
Water purification systems are usually a combination of physical, chemical, and electrical processes that each remove certain impurities. These processes are used in combination to produce water of a desired quality.
Pre-treatment
Much of the water treatment process relies on sensitive membrane technologies, resins, and equipment that can be damaged by certain water impurities, therefore water should be pre-treated to protect equipment. Dissolved ions such as calcium and magnesium can result in scale build up on equipment, and chlorine gas can destroy membranes. Many pre-treatment methods involve the use of polyphosphates, which are a type of chemical that sequesters many minerals and metals (iron, manganese, calcium, magnesium, etc.) by forming soluble complexes. Another pre-treatment method is the use of activated carbon. Activated carbon is a material with a high surface area that binds a wide variety of dissolved gases, chemicals, and bacterial impurities through physical adsorption kinetics.

Ion-Exchange
Ion exchange (IX) is a chemical method used to remove ions from water. For deionization purposes, Ion-exchange columns are composed of resin beads that exchange dissolved ionic impurities for water ions. As the water flows through, positive ions dissolved in the water (e.g., Na+, Ca2+, Al3+) are attracted to the resin, which then releases (exchanges) hydrogen ions into the solutions. Similarly, to remove negative ions (e.g., Cl-, Fl-, NO3-, SO42-), a hydroxyl ion is exchanged. The hydrogen ion from the cation exchanger unites with the hydroxyl ion of the anion exchanger to form pure water. Ultimately, the exchange resin needs to be regenerated when it is saturated with the contaminant ions.
Electrodeionization
Electrodeionization (EDI) is similar to ion-exchange in that dissolved ionic contaminants are exchanged for hydrogen. An internal 'diluting' chamber is filled with similar IX resin that attracts the dissolved ionic contaminants. However, an electrical current is applied and the electric potential draws anions and cations from this diluting chamber into concentrating chambers. Regeneration of the resin is accomplished through electrolysis, splitting the water into its ions H+ and OH-. Thus, electricity is EDI's only consumable. This method of permeate polishing does not produce a hazardous waste stream.
Reverse Osmosis
Osmosis is a natural process whereby molecules that are separated by a semi-permeable membrane move from regions of high concentration to low concentrations to equalize the solute concentrations on the two sides. Reverse osmosis is a process that uses pressure to 'reverse', or work against, this process to ensure that water will flow through the semi-permeable membrane against the osmotic pressure to regions of low solute concentration. This removes many large molecules from solution by ensuring that the solute remains on the pressurized side of the membrane and water molecules can flow through the membrane. When operating at top efficiency, RO can remove particles as small as 0.001 μm.

Membrane Filtration
Membrane filtration shares theoretical similarities with reverse osmosis; however, the primary removal mechanism in membrane filtration is the physical process of size exclusion. As water flows through a semipermeable membrane, higher molecular weight compounds are retained in the retentate, while water and some low molecular weight compounds can pass through to make the permeate. Because this is a physical process based on molecular size, the process can theoretically achieve perfect exclusion of particles irrespective of operational parameters. In contrast, reverse osmosis relies on a diffusive mechanism, making separation efficiency contingent on solute concentration, pressure, and water flow rate.
Ultrafiltration
Ultrafiltration is a sub-category of membrane filtration. The membranes are defined by their molecular weight cut-off (MWCO) and usually operate in cross-flow. Ultrafilters have a MWCO from 5 kDa to 100 kDa. Ultrafilters with a MWCO from 5 to 13 kDa have been shown to remove RNases (< 1 pg/mL), DNases (< 5 pg/mL), and endotoxins (<0.001 EU/mL).
Ultraviolet Treatment
Ultraviolet light is a form of electromagnetic radiation that has wavelengths shorter than visible light, but longer than x-rays. The sun produces UV light in three categories based on their wavelength, UVA ( 315-400 nm), UVB (280-315 nm), and UVC (100-280 nm). Many may be acquainted with UVA and UVB from reading sunscreen bottles, as they are well known for causing sunburns. UVC wavelengths, on the other hand, never reach the earth's surface. Lately though, UVC has been back in the scientific spotlight for its germicidal properties and possibly utility for killing COVID particles2.
UVC, with the shortest wavelength and highest energy, is known to disinfect air, water, and nonporous surfaces. For water treatment, the water flows through a tube and is exposed to UVC light. Wavelengths between 200-300 nM are considered germicidal, with the peak effectiveness at 260 nm. These wavelengths damage both DNA and RNA so that bacteria, viruses, and fungi cannot reproduce and multiply. The amount of damage is proportional to the intensity multiplied by the amount of time the water is exposed.
DEPC-treatment
Another water treatment method that is specific to the removal of nucleases is DEPC (diethylpyrocarbonate). DEPC inactivates enzymes such as nucleases as the nitrogen atoms of the histidine residues react with the DEPC. This comes with some drawbacks as this means that buffers that contain amine compounds cannot undergo DEPC-treatment. Moreover, excess DEPC must be removed using autoclaving, and by-products that are generated during the DEPC-treatment and the autoclaving impair the water quality by creating dissolved organic compounds and increasing the conductivity. Therefore, many laboratories prefer water that has been purified without the use of DEPC.
Norgen's Ultrapure Nuclease-Free Water
Norgen's Ultrapure Nuclease-Free water purification system has been designed in conjunction with experts from Millipore Sigma. The Ultrapure NFW is purified in a two-stage process. The first stage, to produce type II water, includes pre-treatment with activated charcoal and polyphosphate. This water then undergoes ion exchange, electro-deionization, and reverse osmosis to remove contaminants, as well as UV treatment for disinfection. The Pure water then flows to a second system in a clean room with an additional 4 layers of purification. After passing through an additional ion exchange column, the water is then subjected to Ultrafiltration to remove particulates, bacteria, and ionic contaminants down to trace levels. The water is then treated with UV light (182 nm wavelength) to break down organic matter. Lastly, there is a second Ultrafilter for the production of pyrogen-, nuclease- and bacteria-free water at 18.2 MΩ. This water is of utmost purity and suitable for direct use in molecular biology applications such as qPCR and NGS, as well as cell culture and other applications.
The bottles are filled in our clean-room by highly trained technicians, and each lot undergoes rigorous quality control testing. The water purification system is continuously electronically monitored and undergoes regular preventive maintenance. Norgen Biotek is an ISO certified GMP laboratory that has been in business for over 20 years. Use Norgen's Ultrapure Nuclease-Free Water for all your molecular biology needs to ensure accurate and reproducible results. Norgen's Ultrapure Nuclease-Free Water comes in a variety of sizes and formats for all uses.
Related products
| Cat-No. | Item | Size | Price (CHF) |
|---|---|---|---|
| 11-04-02-01 | 10 x 2ml Nuclease Free Water | 20 ml | 14.00 |
| 11-05-01-04 | 1 Liter Nuclease Free Water | 1 l | 41.20 |
| 11-05-01-14 | 300 mL Nuclease Free Water | 300 ml | 12.50 |
| 28002 | Ultrapure Nuclease-Free Water - 100 x 1.25 mL | 100 units | 198.00 |

Norgen Biotek
In addition to the topselling Total RNA Purification Kit line, Norgen also offers collection devices for whole food (cf-RNA/DNA) urine, saliva and more.
About Norgen Biotek Shop for Norgen Biotek products
