What is transfection?
Transfection is a term used to describe the introduction of foreign nucleic acids (DNA or RNA) into eukaryotic cells. typically with the aim of altering cellular properties. It has wide applicability for studying normal cellular processes and the molecular mechanisms of disease. It can be transient, meaning that the introduced genetic material exists in the cells for only a finite length of time, or stable, whereby expression is sustained indefinitely. Stable transfection is limited to DNA, which is either integrated into the cellular genome or preserved as an episomal plasmid. It should be noted that transfection is different from transformation and transduction, which respectively refer to the uptake of foreign nucleic acids by bacteria and the virus-mediated delivery of genetic material.
Although transfection can be achieved using mechanical methods such as electroporation, the use of lipid-based reagents is often preferred due to the lower associated risk of cell damage.
Why might you choose to perform transfection?
One of the main reasons for performing transfection is to recombinantly express a protein of interest exhibiting proper folding and post-translational modifications – something that is unattainable when using bacterial expression hosts. This involves introducing protein-encoding DNA into the cells, where the cellular machinery is then harnessed for protein production. The expressed protein might be purified and used as a control for applications like Western blot or ELISA. Alternatively, it could mediate cellular differentiation, such as through the transfection of reprogramming transcription factors. In addition, this approach underpins many reporter assays, whereby fluorescently-tagged proteins expressed by cells are used for studying protein distribution, dynamics, or action with techniques such as fluorescence microscopy.
Another major application of transfection is its use for inhibiting protein expression through RNA interference (RNAi), which can help to reveal a role for specific proteins in conditions of health and disease.
Transfection is also a key step in the production of biotherapeutics like Humulin®, a recombinant form of insulin that represents the first genetically engineered drug to be approved by the FDA.
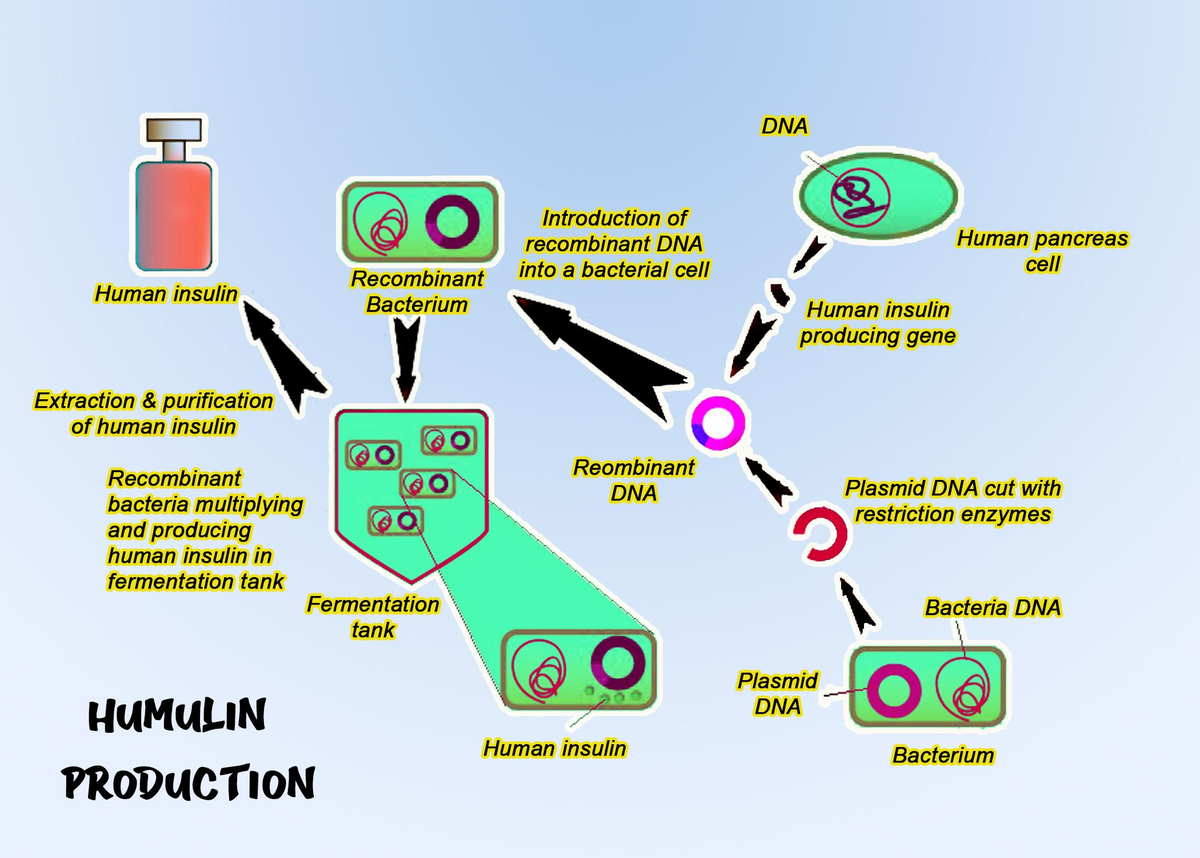
Figure 1: Steps of humulin production 1
1. Synthesis of the gene for human insulin artificially
2. Insertion of human insulin
3. Introduction of the recombinant plasmid into E.coli
4. Culturing recombinant E.coli in bioreactors
5. Extraction of a recombinant gene product from E.coli
6. Purification of humulin
What methods are used for transfection?
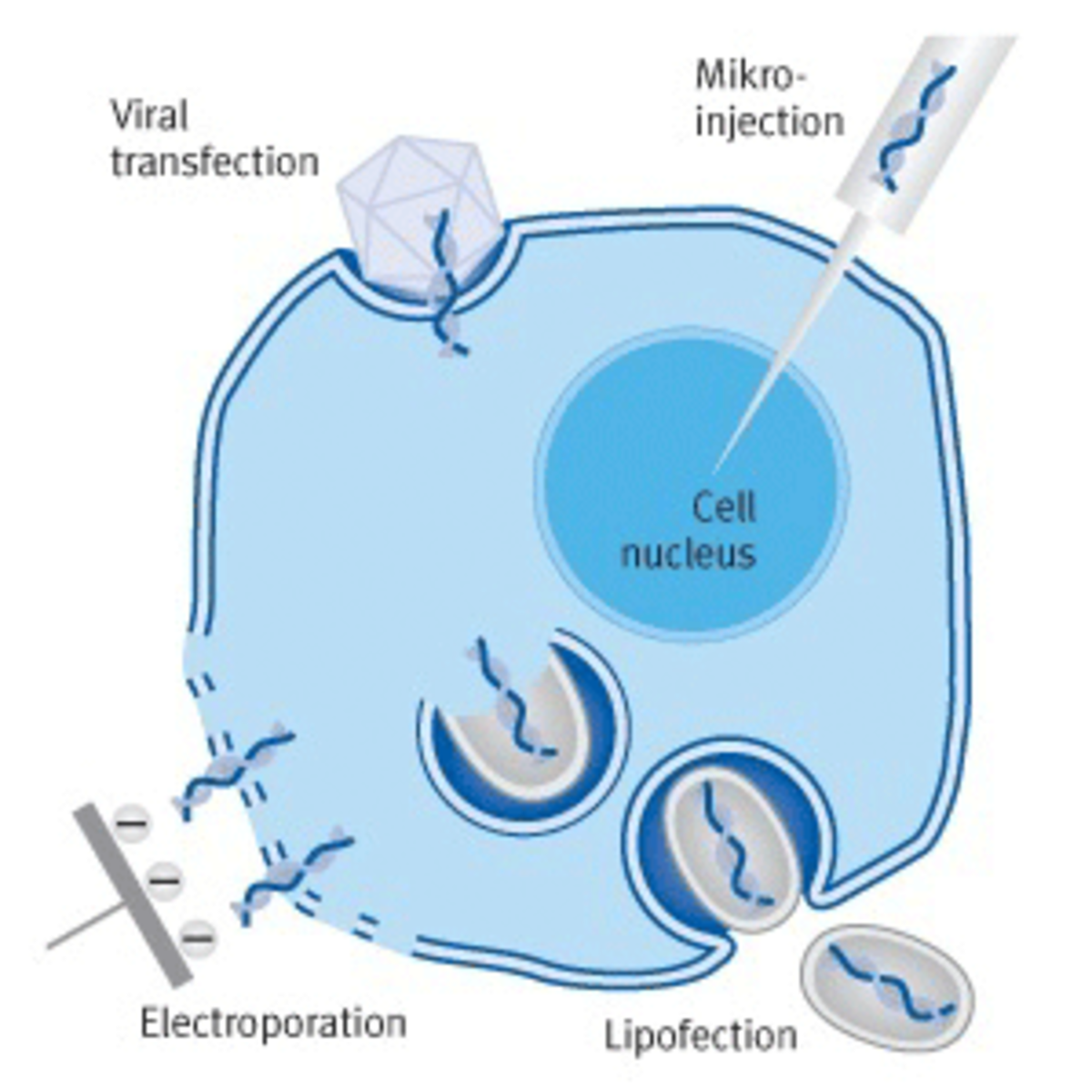
Transfection methods are broadly categorized as either mechanical or chemical. Mechanical methods physically puncture the cell membrane to deliver genetic material into the cytoplasm and/or nucleus. They include microinjection, whereby a fine needle is used for manual gene delivery under a microscope; electroporation, which uses an electrical pulse to form transient pores in the membrane; and particle bombardment, which involves high-velocity delivery of nucleic acids that are bound to nanoparticles. Other well-known mechanical methods are sonoporation, magnetofection, and laser irradiation.
Figure 2: Transfection methods examples 2
Viral transfection, electroporation, microinjection and lipofection
Chemical methods offer a gentler means of transfection. They include the use of cationic polymers such as DEAE-dextran, polybrene, and dendrimers, which are thought to associate with the negatively charged cell membrane to enable nucleic acid uptake by endocytosis. Another approach, termed calcium phosphate co-precipitation, involves mixing nucleic acids with calcium chloride to form a precipitate that is then taken up by cultured cells. However, because these methods have low efficiencies and are cytotoxic to many cell types, they have largely been superseded by reagent-based transfection.
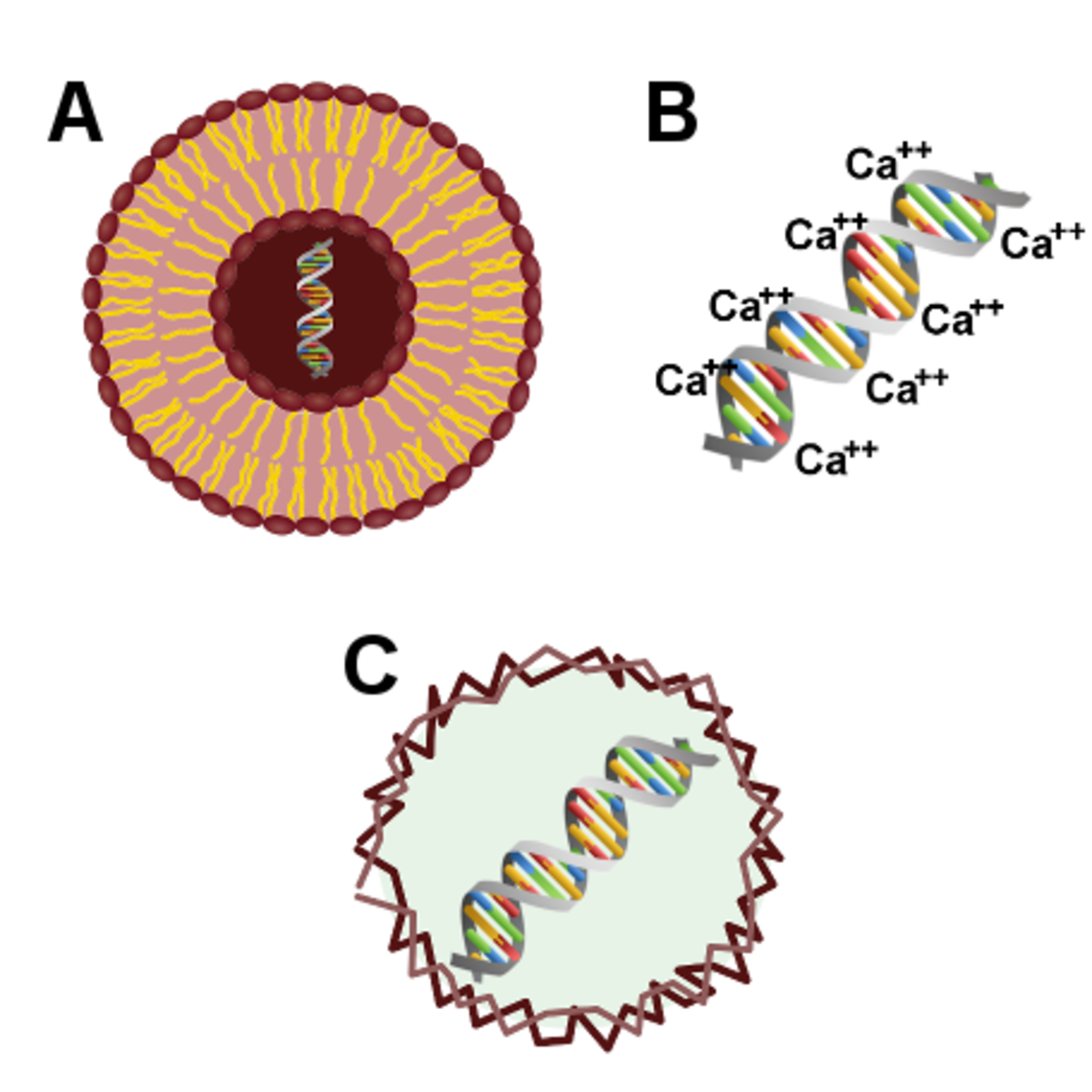
Figure 3: Chemical transfection methods 3
A. Reagent-based transfection, B. Calcium phosphate-based transfection, C. Cationic polymer-based transfection.
The first use of reagent-based transfection reagents for delivering DNA into mammalian cells was described in 1980. It involved forming a colloidal suspension of DNA-containing lipid particles – known as liposomes - for fusion with the cell membrane and demonstrated comparable performance to the calcium phosphate co-precipitation method. Newer reagent-based transfection agents are non-liposomal and designed to address various experimental challenges.
What are the advantages of reagent-based transfection?
A major advantage of reagent-based transfection is that it typically achieves higher transfection efficiencies than other methods. Reagent-based transfection also allows for delivery of a broad range of nucleic acids, spanning small oligonucleotides to large chromosomes, providing increased flexibility for experimental design. Other advantages of reagent-based transfection include its applicability for both transient and stable expression; its broader compatibility with different cell types compared to other methods; and its proven utility for in vivo transfer of nucleic acids.
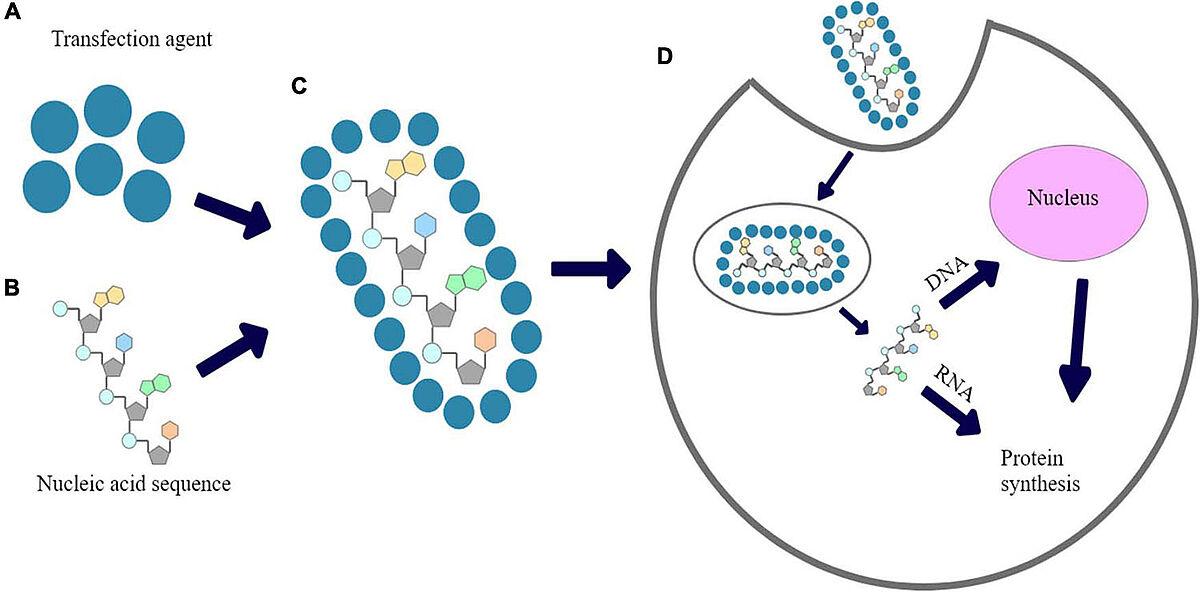
Figure 4: The proposed mechanism of chemical transfection 4
(A) transfection reagent; (B) Nucleic acid sequence; (C) nucleic acid polyplexed with chemical transfection reagent; (D) cellular uptake of polyplex and expression of introduced nucleic acid’s fragment.
What are FuGENE® transfection reagents?
FuGENE® transfection reagents, available through LubioScience, are a range of reagent-based (non-liposomal) products designed to meet various transfection needs - which can vary depending on the cell type and application. Combining high-efficiency transfection with extremely low cellular toxicity, and employing simple, easy-to-use protocols, they currently comprise four main products.
- FuGENE® 6 – the original FuGENE® reagent, designed for delivering DNA of all sizes, suitable for use with over 1000 cell lines
- FuGENE® HD – intended for use with difficult-to-transfect lines such as primary cells, stem cells, and suspension cells, as well for transfecting insect cell lines
- FuGENE® 4K – the most advanced FuGENE® reagent, designed for transfection of both challenging and routine mammalian cell lines, demonstrating improved performance over competitor reagents including Lipofectamine® 3000
- FuGENE® SI – the newest addition to the FuGENE® range, designed for delivery of siRNAs (or similar RNA molecules) into eukaryotic cells to maximize gene knockdown
What are the key considerations for transfection?
As well as selecting a suitable transfection reagent, researchers must consider several other factors when performing transfection experiments. These include cell health, confluency, and passage number, in addition to the quality and quantity of DNA/RNA. As a general guideline, it is recommended to transfect cells at 40 - 80% confluency, keep the number of passages low, and ensure nucleic acids are free of protein and any chemical or microbial contamination. It is also advised to optimize the specific transfection conditions, including the ratio of transfection reagent to nucleic acid, the length of time allowed for transfection to occur, and whether the presence of serum is tolerated.
LubioScience represents some of the most trusted brands in research and works closely with partners such as FuGENE to offer a comprehensive selection of high quality transfection reagents. Contact us today to discuss how we can support your project.
FuGENE® transfection reagents
Want to learn more aboout FuGENE® transfection reagents?
Supplier

FuGENE® - See the unseen
FuGENE® offers a full portfolio of best-in-class transfection reagents
References
- Figure 1 https://www.vedantu.com/question-answer/the-steps-involved-in-the-production-of-humulin-class-12-biology-cbse-5faf13e31850a26db6d6882d
- Figure 2 https://www.biontex.com/europe/transfection/
- Figure 3 https://theory.labster.com/transfection-methods/
- Figure 4 https://www.frontiersin.org/articles/10.3389/fbioe.2021.701031/full