Next-Gen ChIP-Seq: Genome-Wide Single-Cell Analysis with Antibody-Guided Chromatin Tagmentation Methods
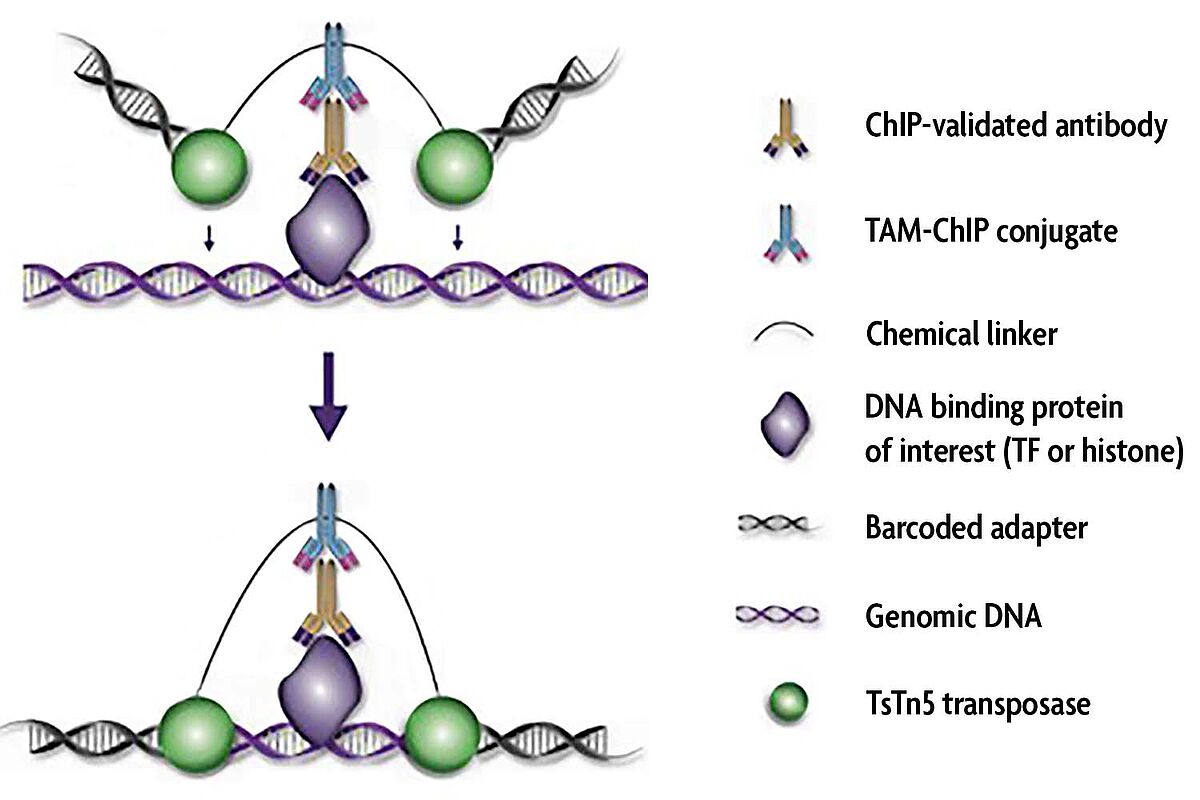
As amazing as ChIP-Seq assays are, most ChIP protocols do not perform well with low numbers of cells. New methods are being developed to improve the efficiency of epigenomic analysis, including some reports that analyze histone modifications at the single-cell level. Many of these methods are variations on Active Motif’s patented TAM-ChIP™ technology, which utilizes antibody-mediated tagmentation to generate NGS libraries.
How Can You Do Single-Cell ChIP-Seq Assays?
Single-cell genomic analyses have come a long way in a short amount of time. There are already robust assays that enable performing methods like RNA-Seq (transcriptome analysis) and ATAC-Seq (open chromatin analysis) on a single-cell level. These technologies are showing great promise for the analysis of clinical samples and samples that contain heterogeneous populations of cells, such as cancer.
However, it remains difficult to specifically investigate epigenetic profiles like histone modifications on a genome-wide scale at the single-cell level.
In particular, ChIP-Seq assays are generally not compatible with single-cell analysis because the chromatin preparation and sonication steps in the chromatin immunoprecipitation workflow require relatively high numbers of cells for optimal results.
Therefore, researchers are developing methods to take advantage of the good parts of the ChIP workflow (using high-quality antibodies that are extremely specific) while also eliminating the problematic parts (nuclear isolation and sonication that can be tedious and lead to sample loss). These newer methods often combine the library preparation and immunoprecipitation steps into a single step.
Combining ChIP with Tagmentation to Improve Sensitivity
The first method that started this new low cell ChIP-Seq movement was the patented TAM-ChIP™ technology from Active Motif. The TAM-ChIP method utilizes secondary antibodies conjugated to the TsTn5 transposase enzyme and Illumina-compatible NGS library adapters so libraries can be generated directly as part of the immunoprecipitation step rather than requiring purification of the ChIP DNA prior to library preparation.
The core technology used in TAM-ChIP has been called various other names, including CUT&Tag, ChIPmentation, TAF-ChIP, ChIL-Seq, ACT-Seq, CoBATCH, and more.
All of these methods generally rely on antibody-mediated tagmentation using the Tn5 transposase to introduce NGS library adapters.
The beauty of these approaches is that they are more compatible with smaller numbers of cells, which allows the analysis of clinical samples where limited sample sizes are available as well as improved analysis of heterogeneous cell or tissue populations.
Is Genome-Wide Single-Cell Histone Analysis Really Possible?
There have been several publications that present genome-wide single-cell histone modification data, but these assays are currently only being performed by a few of the most skilled epigenetics researchers and it’s not yet clear how reproducible the data are.
As the technology used in biomedical research improves, more and more researchers are starting to do single-cell assays. New methods for single-cell epigenomic studies are also becoming more common.
Some of the recent single-cell epigenomic studies are listed below.
Single-Cell CUT&Tag
CUT&Tag stands for Cleavage Under Targets and Tagmentation and was developed in the Henikoff lab at the Fred Hutchinson Cancer Research Center. A recent paper in the journal Nature Communications published single-cell CUT&Tag (scCUT&Tag) data for the histone modifications H3K27me3 and H3K4me2 using the K562 cell line.
Single-Cell ChIL-Seq
A team of scientists from different research institutes in Japan developed a method called ChIL-Seq, which stands for chromatin integration labeling followed by sequencing. The researchers published single-cell ChIL-Seq data in the journal Nature Cell Biology and they were able to generate epigenomic data at the single-cell level for the histone modifications H3K4me3, H3K27me3, and H3K27Ac in the C2C12 cell line.
Single-Cell ACT-Seq
Antibody-guided chromatin tagmentation sequencing (ACT-Seq) is another transposase-based method for single-cell epigenomic analysis. ACT-Seq and a single-cell version called iACT-Seq (for indexing ACT-Seq) were developed by a team of researchers at the National Institutes of Health. They published their single-cell iACT-Seq results in the journal Nature Communications and were able to generate single-cell data for the histone modification H3K4me3 in HEK293 cells.
Single-Cell CoBATCH Assays
CoBATCH stands for combinatorial barcoding and targeted chromatin release and was developed by researchers in China. They published results from their single-cell CoBATCH assays in the journal Molecular Cell and were able to generate single-cell data for the histone modification H3K27Ac in mouse embryonic stem cells (ESCs) and single endothelial cells derived from 10 different mouse embryonic organ tissues.
Summary: Antibody-Mediated Tagmentation Methods Might Be the Future of ChIP
Many discoveries have been made over the past few decades using ChIP and ChIP-Seq assays. We now have a deep understanding of the genome-wide epigenomic profiles of many different histone modifications, and we have also learned a lot about the genome-wide DNA-binding patterns of transcription factors and other proteins that associate with chromatin.
One of the limitations of chromatin immunoprecipitation assays has historically been that these methods are not always robust with small numbers of cells or a small amount of tissue due to sample loss during the chromatin preparation and sonication steps.
Those previous limitations are starting to fade away with the development of new antibody-mediated tagmentation methods can combine the specificity of antibodies with the ability of transposase enzymes to be conjugated to NGS adapters to directly generate sequencing libraries at genomic sites bound by the antibodies.
TAM-ChIP and other related tagmentation methods might represent the future of epigenomic assays, and we’re excited to see what discoveries will be enabled by these technologies.
Want regular NGS news? Then subscribe to our NGS newsletter!