IBA Lifesciences GmbH is a biotechnology company providing products for life science research in academia and industry. They develop high performance research tools for cell and protein isolation based on their proprietary Strep-tag® technology. Situated in Göttingen, Germany, they have built a network of worldwide distributors making our products available in over 40 countries across 5 continents.
Portfolio
|
|
Strep-tag® technology
High performance research tools for cell and protein isolation
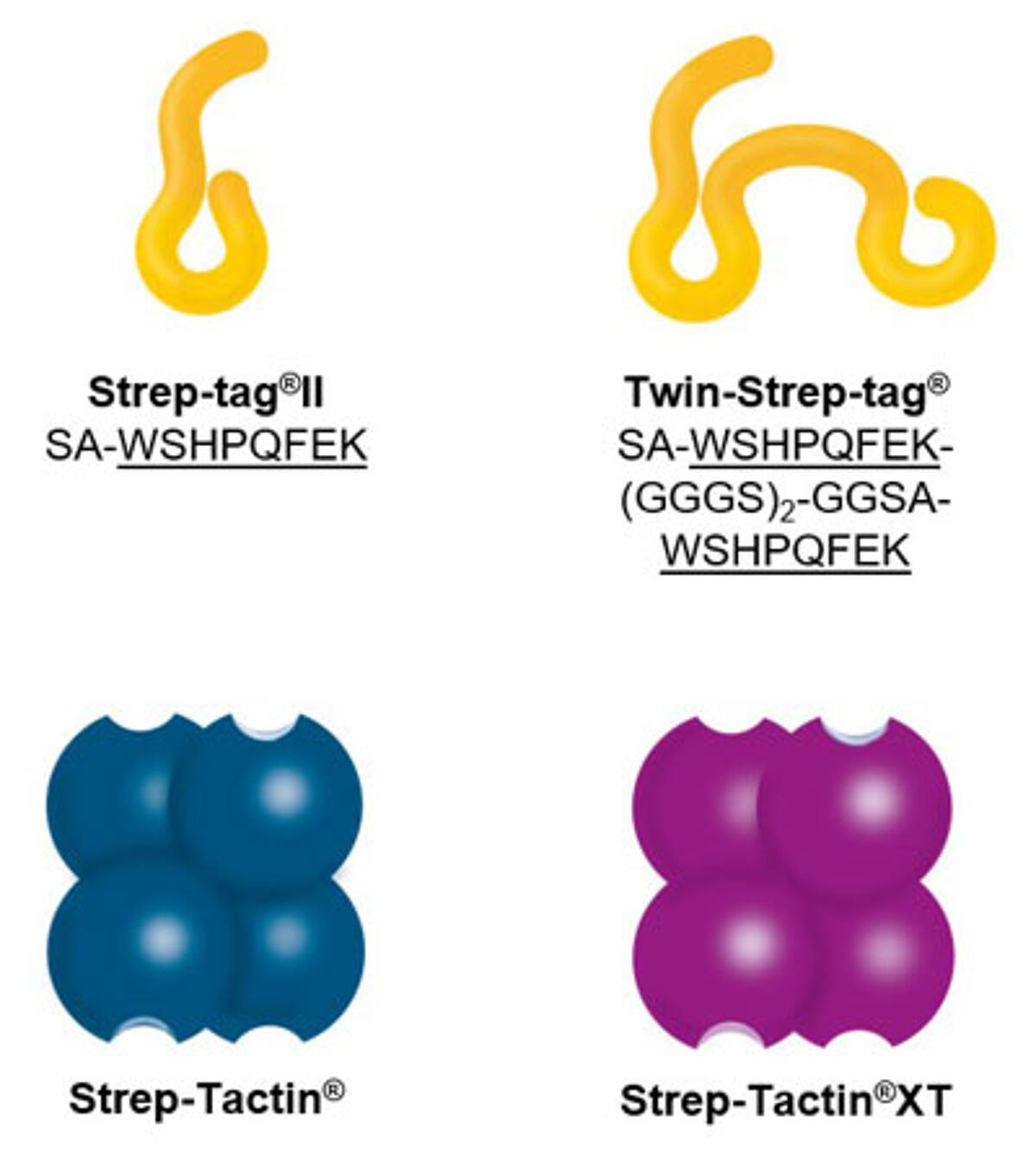
Components of the Strep-tag® technology
Our proprietary Strep-tag® technology exploits one of the strongest non-covalent interactions in nature: the interaction of biotin and streptavidin. The system is based on the highly selective and easily controllable interaction between the synthetic Strep-tag®II peptide and the specially engineered streptavidin, called Strep-Tactin®, which is one of the most stable proteins known. The Strep-tag®II binds specifically to the engineered streptavidins, Strep-Tactin® and Strep-Tactin®XT, by occupying the binding pocket of the natural ligand biotin. Hence, the interaction is easily reversible by excessive addition of the competitor.
Two affinity tags – two streptavidin derivatives: The Strep-tag®II consists of eight amino acids (Trp-Ser-His-Pro-Gln-Phe-Glu-Lys), whereas the Twin-Strep-tag® includes this motif two times in series connected by a linker and is accordingly composed of 28 amino acids. Both exhibit intrinsic, although unequal, affinity towards the streptavidin derivative Strep-Tactin® and its successor Strep-Tactin®XT: The binding affinity of Strep-tag®II to Strep-Tactin® (1µM) is nearly 100 times higher than to streptavidin. A further improvement was achieved by the development of Strep-Tactin®XT, which shares a nM affinity with the Strep-tag®II and a pM affinity with the Twin-Strep-tag®.
As a result of the differences in binding strength among the possible tag-ligand combinations, the Strep-tag® system has become established as a universal tool for isolation of proteins and cells.
- Fused to recombinant proteins, the Strep-tag® enables efficient one-step purification on immobilized Strep-Tactin®.
- When fused to antibody-derived Fab fragments or nanobodies, Twin-Strep-tag® binds to multimerized Strep-Tactin® allowing capturing and releasing of target cells based on their surface-marker or antigen-specificity.
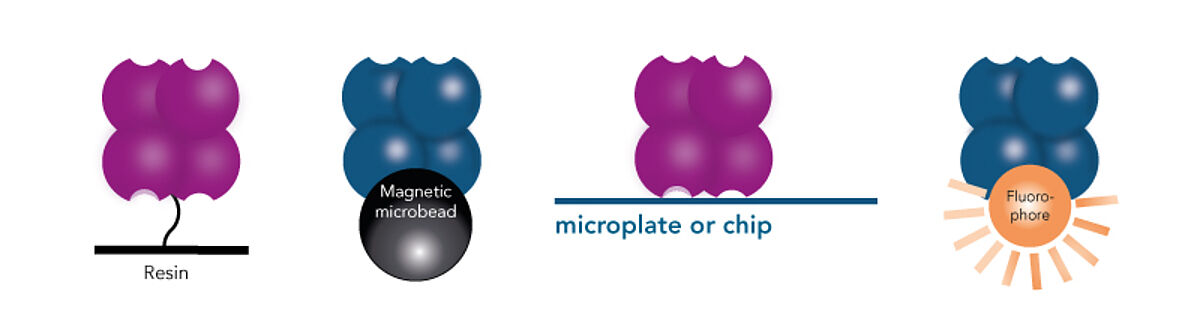
- Strep-Tactin® (or Strep-Tactin®XT), on its turn, can be conjugated to microplates, fluorophores or chips, allowing a wide range of analytical applications after isolation of target material, such as detection, immobilization and interaction studies.
Top Sellers
Streptavidin
Efficient immobilization of biotinylated analytes
Streptavidin is a tetrameric protein composed of identic subunits. Each subunit specifically binds one biotin molecule with fM affinity, which makes it one of the strongest non-covalent interactions known. The preparation contains an N- and C-terminal shortened variant (core streptavidin) with improved properties regarding homogeneity, solubility, resistance towards proteolytic degradation, and accessibility of the biotin binding pocket as compared to native streptavidin. The streptavidin:biotin system is widely used for immobilization and detection of biotinylated molecules, like proteins and nucleic acids.
StrepMAB-Classic HRP
Conjugated monoclonal antibody to detect Strep-tag®II and Twin-Strep-tag® fusion proteins
StrepMAB-Classic HRP is a highly specific monoclonal antibody conjugated to horseradish peroxidase for the detection of Strep-tag® and Twin-Strep-tag® fusion proteins. It can be used for Western Blots, ELISA, immunohistochemistry and immunocytochemistry. StrepMAB-Classic HRP is suitable for chromogenic and chemiluminescent reactions.
Strep-Tactin®XT 4Flow® high capacity FPLC column
FPLC column to purify Strep-tag®II or Twin-Strep-tag® fusion proteins
Strep-Tactin®XT 4Flow® high capacity FPLC columns contain 4% agarose resin, coupled to Strep‑Tactin®XT. The columns can be used for fully automated purification of Strep-tag®II or Twin-Strep-tag® fusion proteins with HPLC/FPLC devices, like Äkta®. The resin is suitable for all protein classes, especially low abundant or challenging proteins. The low concentration of agarose is suitable for the purification of targets with high molecular weights. In comparison to Strep-Tactin® 4Flow® resin, this high capacity variant has higher density of Strep-Tactin®XT, resulting in twice as much purified protein. The resin has broad pH compatibility, excellent pressure stability, strong binding affinity and delivers high target purity (> 95 %).
For the purification of multimeric proteins, we recommend using Strep-Tactin® Superflow® high capacity FPLC columns instead, since the increased avidity might influence elution efficiency. If you want more information, check out our resin specification overview.
Please note that this product was previously called Strep-Tactin®XT 4Flow® high capacity cartridge.
Strep-Tactin®XT 4Flow® high capacity resin
50% suspension to purify Strep-tag®II or Twin-Strep-tag® fusion proteins
Strep-Tactin®XT 4Flow® high capacity is perfectly suited for purifying Strep-tag®II or Twin-Strep-tag® fusion proteins. This resin offers excellent pressure stability, pH compatibility, and target purity (> 95%). Additionally, this resin is compatible with a broad range of reagents.
Its high binding affinity makes it ideal for low abundant, high-expressing and challenging proteins. A low concentration of agarose also facilitates the purification of large proteins (>90 kDa). When working with, for example, multimeric proteins, choosing a resin with the Strep-Tactin® ligand is recommended.
Strep-Tactin®XT 4Flow® high capacity resin has a higher density of immobilized Strep-Tactin®XT than the classic Strep-Tactin®XT 4Flow®and can thus can bind more protein.
As the resin is optimized for column affinity chromatography, rather than batch purification, the suspension can be used to pack gravity flow columns.
Strep-Tactin®XT 4Flow® high capacity column
Gravity flow column to purify Strep-tag®II or Twin-Strep-tag® fusion proteins
Strep-Tactin®XT 4Flow® high capacity columns are applicable to the purification of Strep-tag®II or Twin-Strep-tag® fusion proteins via gravity flow. It is recommended for proteins of all classes, especially low abundant, large, and highly expressed proteins, due to its pH compatibility, target purity (> 95%), and high binding affinity. Multimeric protein complexes might bind too strongly to our Strep-Tactin®XT 4Flow® high capacity resins, preventing efficient elution. Strep-Tactin® resins are recommended for these proteins.
With Strep-Tactin®XT 4Flow® high capacity, denaturing agents such as urea can be used. In comparison to Strep-Tactin®XT 4Flow®, Strep-Tactin®XT 4Flow® high capacity has a higher density of immobilized Strep-Tactin®XT, which leads to a higher yield of purified protein.
Purify large samples faster with our WET FRED.
MagStrep® Strep-Tactin®XT beads
Magnetic beads (5% suspension) to purify Strep-tag®II or Twin-Strep-tag® fusion proteins
MagStrep® Strep-Tactin®XT beads can be used for the purification of strep-tagged fusion proteins in batch or high-throughput format.
For cell purification we offer specific magnetic beads with a smaller diameter (1µm) and for biotinylated proteins we recommend our MagStrep® Strep-Tactin® beads.
MagStrep® Strep-Tactin®XT beads show low non-specific protein binding due to their agarose surface and high specificity of Strep-Tactin®XT towards Strep-tag. This results in outstanding target protein purity and makes the MagStrep® Strep-Tactin®XT beads the ideal tool for protein isolation and complex identification via pull-downs. MagStrep® Strep-Tactin®XT beads are suitable for fast small-scale purifications in reaction tubes as well as 96-well plates.
The beads can be easily and swiftly separated with a magnetic separator. This eliminates the need for centrifugation and reduces the risk of disturbing the pellet or loss of beads within the wash step to zero. The elution of target proteins or complexes can be triggered under mild conditions with the biotin-containing Buffer BXT or under denaturing conditions by boiling in SDS gel loading buffer.