Why use gel documentation systems?
Gel documentation systems or GelDocs are an integral part of any laboratory, no matter whether it is a clinical, diagnostic or research laboratory. A GelDoc system consists of three main parts: a transilluminator, which provides the appropriate excitation for the dyes used, a casing to shield from external light and protect the user from UV irradiation, and a camera to capture images and enable data storage.
GelDocs enable the measurement and recording of labelled DNA, RNA and protein fragments that have been separated according to their size and charge. Various applications employ GelDocs, from monitoring cloning reactions to size selection of genomic DNA, RNA or final libraries in next-generation sequencing (NGS). Protein-based applications of GelDocs use white LED light transillumination to analyse samples stained with protein stains. GelDocs with white LED epi-illumination permit the live assessment of microorganisms on semi-solid and opaque media, such as Petri dishes.
CCD cameras incorporated into GelDocs allow the visualisation of samples and the acquisition of high-quality images. These digital images make data presentation and data transfer easy. Integration of GelDoc data into laboratory information management systems (LIMs) enables sample processing and downstream analysis to be tracked, monitored and archived.
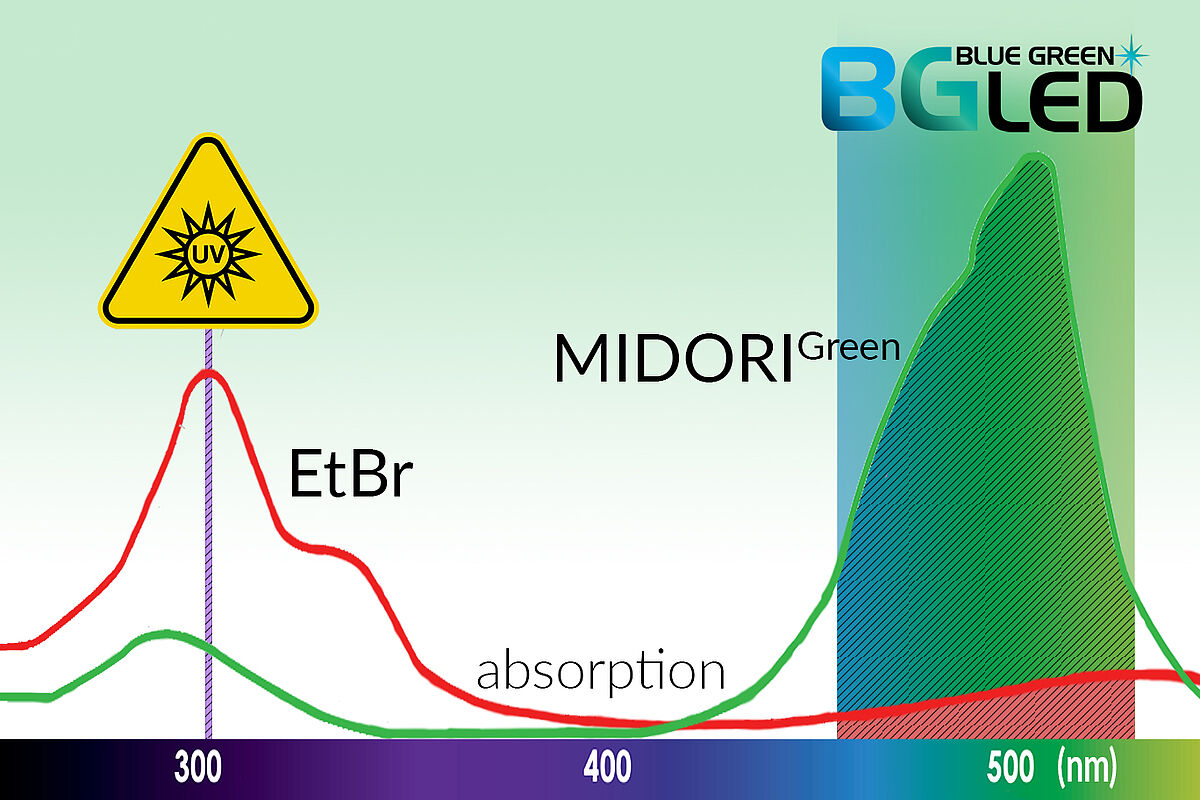
Modern GelDoc systems are available with light sources of differing wavelengths, which are optimised for specific workflows. GelDoc systems utilising blue (408 nm) or blue/green (470–520 nm) LED technology instead of UV light at 302 nm safeguard DNA integrity and ensure a safe working environment for laboratory users. Unlike transilluminators with flat windows below which are UV sources, GelDocs systems encase the light source, and users avoid UV exposure.
Light sources used for GelDoc systems
UV light sources
A traditional way to visualise DNA is to excite fluorescent nucleic acid dyes using UV light sources and then monitor their fluorescence emission spectra. The advantages of this technique are that fluorescent dyes are stable over extended periods, and UV light sources are inexpensive. However, UV light causes lesions and mutations in DNA1. A 30-second exposure to UV light results in significant decreases in cloning efficiencies compared to blue or blue/green light2. UV damage to DNA integrity can significantly affect applications that require the excision of DNA fragments from gels for cloning experiments, negatively influencing downstream applications. Therefore, using alternatives to UV light sources can improve the performance of experiments and allow the user to have more confidence in the results generated.
Blue LED technology
Alternatives to UV light excitation include exciting dyes with blue light (408 nm), which reduces the risk of eye damage and cancer to users by eliminating UV exposure. In addition, blue light is less damaging to DNA than UV light (302 nm) but can still efficiently excite commonly used nucleic acid stains. Blue light technology is not recommended for visualising traditional dyes such as ethidium bromide as their peak excitation is around 302 nm. Alternative dyes compatible with blue LED technology are available and contain chromophores more suited to excitation by light in the blue spectrum. Such dyes include the Midori Green dyes (discussed below), SYBR dyes and others.
Blue/green LED technology
By extending the excitation range to cover the blue/green light region (470–520 nm), a wider range of dyes can be utilised to visualise DNA. Companies like Nippon Genetics Europe, whose products are available from LubioScience, offer blue/green light sources (Table 1) that provide superior sensitivity to both green and red dyes. An additional advantage of blue and blue/green light technologies is that dyes are excited in the visible range, thereby avoiding DNA damage that occurs with UV light.
Compatible dyes
Dyes compatible with UV light sources
The traditional stain utilised with UV detection is ethidium bromide, which can either be added to the running buffer when samples migrate through the gel or used in post-staining by soaking the gel following migration. A DNA intercalator, ethidium bromide, inserts itself between the base pairs in the double helix. The fluorescent complex formed between ethidium bromide and DNA exhibits a 20- to 25-fold fluorescence enhancement, observed following UV excitation3. Ethidium bromide is a sensitive and inexpensive fluorescent stain but is carcinogenic and fatal if inhaled4 and so has significant handling-related drawbacks and strict disposal regulations. In addition, its visualisation using high-energy short wavelength UV light can harm users5,6.
Dyes compatible with blue and blue/green light
Using dyes compatible with blue/green light as alternatives to UV-excitable dyes - The drawbacks of using UV light sources and ethidium bromide have led to the development of dyes and light sources operating in the blue and blue/green regions of the spectrum.
Advantages of dyes compatible with blue and blue/green light sources:
- Eliminate harmful exposure to the highly mutagenic ethidium bromide and UV light.
- Reduce ethidium bromide disposal costs.
- Use a wider selection of dye choices and protocols.
- Secure downstream applications without fear of degraded genetic material.
Dyes compatible with blue and blue/green light sources:
- Midori Green Dyes (Midori Green Advance, Midori Green Xtra and Midori Green Direct)
- GelRed®
- SYBR®
Some dyes compatible with blue and blue/green technology exhibit higher backgrounds and can produce a speckled effect on the surface of the gel because of fungi, bacteria that fluorescence at the same wavelength. Reusing these dyes is also discouraged as it significantly lowers sensitivity. However, the Midori Green dyes provide strong fluorescence signals with low background staining. Most importantly, Midori Green dyes are non-intercalating and non-carcinogenic and show no mutagenic effect using the Ames test or toxic effects7–9. By binding directly to the nucleic acid backbone, the Midori Green dyes cause less enzymatic inhibition than ethidium bromide and SYBR Green10 and are available for different experimental setups.
The Midori Green Xtra is a highly sensitive in-gel and post-staining fluorescent dye for DNA and RNA. The low background fluorescence produced makes identifying low amounts of DNA very straightforward.
The Midori Green Advance is optimised to bind and visualise small DNA fragments with high sensitivity. Also used for in-gel and post-staining, the Midori Green Advance produces a bright signal when excited by UV light or blue/green light and can be used at a dilution factor of as high as 1:25000.
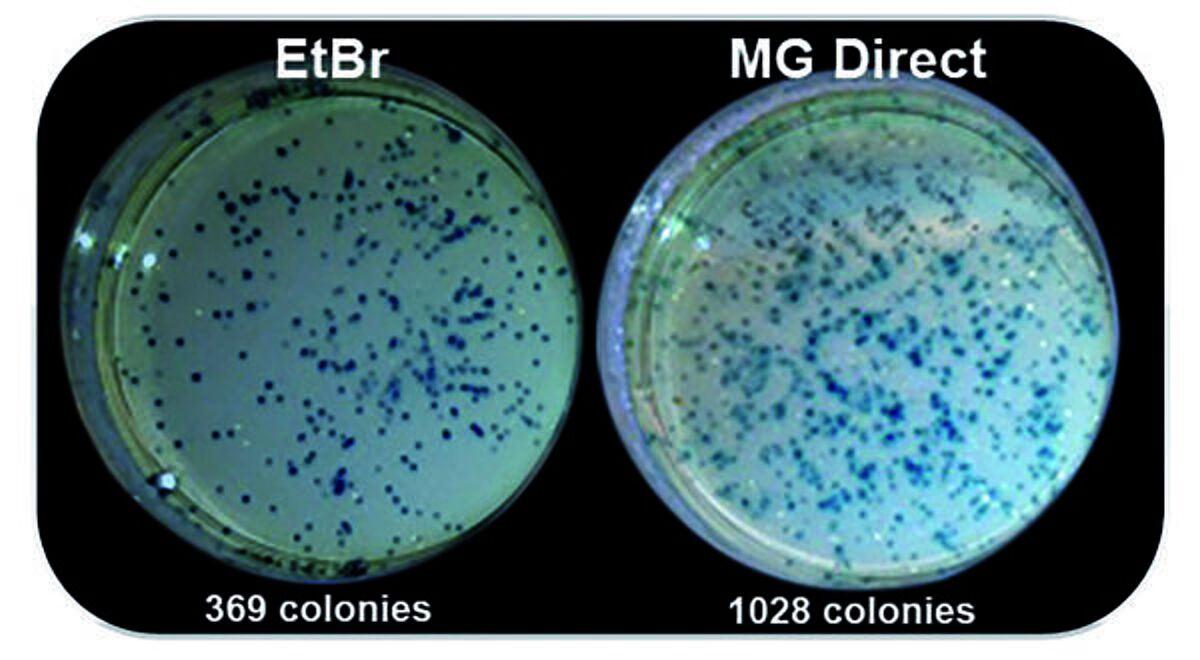
Midori Green Direct stain can be added directly to your DNA samples as a loading dye as opposed to the gel matrix or running buffers, saving the user time whilst benefiting from strong signals with low background staining. Midori Green Direct stain is less damaging to DNA and can be successfully used to assess cloning efficiency2.
In combination with blue and blue/green light technology, the range of Midori Green dyes offers the following solutions:
- Higher cloning efficiency.
- Avoid harmful exposure of users to UV light.
- Non-toxic alternative to ethidium bromide.
Gel documentation systems for diverse applications
Nippon Genetics Europe offers Gel documentation systems with a range of specifications, ensuring the optimum solution is available for every laboratory, regardless of size, logistical or application requirements (Table 1). The FastGene® FAS-V GelDoc is a sophisticated GelDoc system that allows high-throughput analysis whilst enabling a simple user interface with a touchscreen display and on-site imaging editing functions.
For applications requiring mobile imaging, the FastGene® FAS-Nano is recomended, the most portable GelDoc system on the market. Sold with a Nano Amber Shield, the FAS-Nano uses the camera of a phone or tablet to image gels, allowing data to be easily generated at different locations. Table 1 below allows you to select the solution for your laboratory setup.
Table 1: Comparison table
| High-End | Mid-Range / Compact | Compact | Compact | |
|---|---|---|---|---|
| Product name | FAS-DIGI PRO | FAS-DIGI Compact | FAS-BG LED BOX | FAS-Nano |
| Overview | The FastGene® FAS-DIGI PRO is a powerful imaging system able to simultaneously image multiple gels because of its large, illuminated imaging area (26 cm x 21 cm). | The FastGene® Fas-DIGI Compact is a compact stand-alone imaging system able to connect to a tablet via the app. | The FastGene® FAS-BG LED BOX is a compact stand-alone imaging system with a touchscreen display. | The FastGene® FAS-Nano is the smallest, most lightweight and most portable doc system on the market. Its small footprint makes it ideal for mobile testing facilities using a smartphone. |
| Technology | Blue/Green LED technology (470-520 nm) | Blue/Green LED technology (470-520 nm) | Blue/Green LED technology (470-520 nm) | Blue/Green LED technology (470-520 nm) |
| Additional features | • White LED light for visualising protein gels. • Amber View Shield | • Amber Filter window to facilitate cutting of desired DNA bands for further applications. | • White LED plate for visualising protein gels. • White Epi light for visualising opaque surfaces (Petri dishes and membranes) • Amber View Shield | • Nano Amber Shield |
| Dye compatibility | MIDORIGreen Dyes, GelRed® and SYBR® and red dyes (ethidium bromide) | MIDORIGreen Dyes, GelRed® and SYBR® and red dyes (ethidium bromide) | MIDORIGreen Dyes, GelRed® and SYBR® and red dyes (ethidium bromide) | MIDORIGreen Dyes, GelRed® and SYBR® and red dyes (ethidium bromide) |
| User interface and image editing | • Connects to a computer • Easy-to-use image software (NIPPON Genetics Camera Studio v1.0) includes image acquisition features. | Connects to a tablet via an app. | • 5" Colour LCD Touchscreen display • HDMI port allows display on an external screen. | Compatible with any phone or tablet with a camera. |
| Camera | 24 M Pixel camera, Light-sensitive | 24 M Pixel camera | 8 M Pixel camera | Uses the camera from a smartphone or tablet |
| Illuminator size | 26 x 21 cm | 26 x 21 cm | 16 x 11.5 cm | 10 x 10 cm |
| Dimensions (H x L x W) | 57 x 35 x 32.5 cm | 50 x 35 x 32.5 cm | 23 x 25.4 x 20.7 cm | 12.8 x 21.6 x 16.8 cm |
| Weight | 14 kg | 7.4 kg | 3.2 kg | 1.2 kg |
| Catalogue number | GP-07LED | GP-08LED | GP-04LED | GP-06LED |
Conclusions
GelDoc systems allow users to visualise nucleic acids and proteins and subsequently generate and analyse digital images. This allows scientists to scrutinise high-quality results and reach conclusions faster. Employing blue/green light technology creates safer laboratory environments for users and safeguards DNA integrity for downstream applications by avoiding UV exposure. LubioScience provide gel documentation systems with blue/green light technology in various specifications to suit multiple laboratory needs. In combination, The MIDORI range of dyes, also available from LubioScience, are non-carcinogenic nucleic acid stains that give bright signals when excited by blue/green light offering effective alternatives to ethidium bromide. With this combination, LuboScience can meet all your nucleic acid visualisation needs.
Read more about Midori Green here.
Suppliers

Nippon Genetics - reagents & tools for Molecular Biology
Midori Green safe DNA stains, DNA/RNA visualization, gel documentation, RNA & DNA purification, cell freezing & storage, filter tips & PCR tubes & plates.
References
- Rastogi RP, Richa, Kumar A, Tyagi MB, Sinha RP. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J Nucleic Acids. 2010;2010. doi:10.4061/2010/592980
- Nippon Genetics Europe. Influence of Ethidium Bromide and UV-light on the cloning efficiency - Improved cloning efficiency with Midori Green Direct and Blue LED light. Published 2014. Accessed September 9, 2022. www.lubio.ch/assets/PDFs/Nippon_Midori_Green_Cloning_Efficiency.pdf
- Lepecq JB, Paoletti C. A fluorescent complex between ethidium bromide and nucleic acids. Physical-Chemical characterisation. J Mol Biol. 1967;27(1). doi:10.1016/0022-2836(67)90353-1
- Oakland University. Ethidium Bromide . Oakland laboratory safety. Published 2022. Accessed September 8, 2022. oakland.edu/Assets/upload/docs/LabSafety/EBrwFilters.pdf
- Lunn G, Sansone EB. Ethidium bromide: Destruction and decontamination of solutions. Anal Biochem. 1987;162(2). doi:10.1016/0003-2697(87)90419-2
- Ohta T, Tokishita SI, Yamagata H. Ethidium bromide and SYBR Green I enhance the genotoxicity of UV-irradiation and chemical mutagens in E. coli. Mutat Res Genet Toxicol Environ Mutagen. 2001;492(1-2). doi:10.1016/S1383-5718(01)00155-3
- Nippon Genetics Europe. Midori Green Direct DNA Stain Safety Test Report. Nippon Genetics Europe. Published 2022. Accessed September 20, 2022. www.lubio.ch/assets/PDFs/Nippon_Midori_Green_Direct_Safety_Report.pdf
- Nippon Genetics Europe. Midori Green Advance DNA Stain – Safety Repor. Nippon Genetics Europe. Published 2022. Accessed September 20, 2022. www.bulldog-bio.com/wp-content/uploads/2019/03/midorigreenadvancesafetyreport.pdf
- Nippon Genetics Europe. Midori Green Xtra DNA Stain – Safety Report. Nippon Genetics Europe. Published 2022. Accessed September 20, 2022. www.nippongenetics.eu/app/uploads/Midori-Green-Xtra-Safety-Report.pdf
- Wittwer CT, Herrmann MG, Moss AA, Rasmussen RP. Continuous Fluorescence Monitoring of Rapid Cycle DNA Amplification. Biotechniques. 2013;54(6). doi:10.2144/000114043