Mounting is the final protocol step in immunocytochemistry (ICC) and immunohistochemistry (IHC) workflows. It serves to protect sample material and enhance image quality, and can be especially important for immunofluorescence (IF) imaging. Before discussing the purpose of mounting and how it should be done, we describe the different techniques of cell and tissue staining.
What is the difference between ICC, IHC, and IF?
The terms ICC, IHC, and IF are sometimes used interchangeably, yet they are fundamentally different techniques. ICC is the process of immunostaining intact cells which have either been grown in a monolayer or obtained as a suspension (e.g., non-adherent cell cultures, blood smears, or swabs) before being deposited onto glass slides. IHC is instead the process of immunostaining tissue samples, which may be either frozen or formalin fixed paraffin-embedded (FFPE). While both ICC and IHC have traditionally relied on chromogenic detection, whereby an enzyme such as horseradish peroxidase (HRP) converts a substrate such as 3,3'-diaminobenzidine (DAB) into a colored precipitate at the antigen site, IF imaging is increasingly popular as it allows for multiplexing and does not require that signal development be subjectively monitored and stopped.
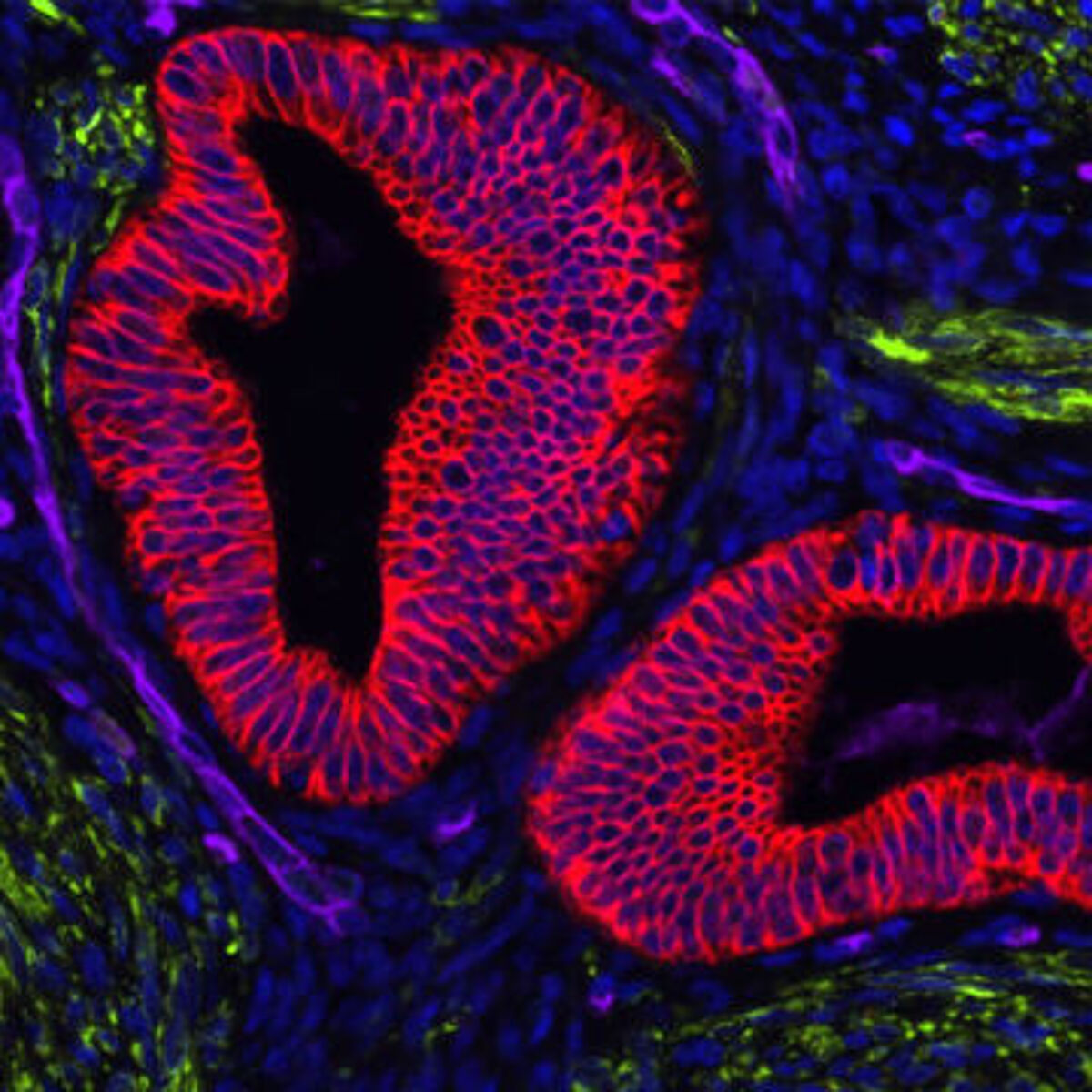
How does the staining protocol vary for ICC and IHC?
A typical ICC protocol begins with growing cells on sterile glass coverslips before fixing in paraformaldehyde or methanol. Paraformaldehyde fixed samples may then need to be permeabilized (e.g., using 0.5% Triton X-100 or Tween-20) to allow antibodies to access intracellular antigens. Next, the samples are blocked (e.g., using 5% normal serum or BSA) and incubated with antibody reagents. These could be either labeled primary antibodies, or a combination of unlabeled primary antibodies and labeled secondary antibodies with a wash step in between antibody incubations. After washing to remove any unbound antibodies, the samples are counterstained (e.g., using DAPI or Hoechst to detect nuclei), then each coverslip is inverted onto a drop of mounting media on a glass slide, ready for imaging.
The IHC protocol varies depending on whether frozen or FFPE tissue is used, but begins with applying freshly sliced sections onto glass slides and allowing them to air dry. In the case of frozen tissue, the protocol is similar to that of ICC. However, for FFPE tissue, additional sample processing steps are required. Specifically, the sections must be deparaffinized and rehydrated (by sequential immersion in xylene, decreasing concentrations of ethanol, then deionized water), and must usually be subjected to antigen retrieval prior to (optional) permeabilization and blocking. Antigen retrieval removes protein cross-links caused by formalin fixation to make epitopes available for antibody binding. It may be heat-induced, or involve the use of proteolytic enzymes, and must be carefully optimized (including incubation time, temperature, and pH) for the system being studied. At the end of this method, again your sample is inverted onto a drop of mounting media.
Fortunately, for the last step it does not mater which method you used. Mounting your cells or tissues remains the same.
What is the purpose of mounting?
Mounting is the final step in the ICC/IHC workflow. It protects the sample by holding it in place during imaging (which avoids damage due to shearing or inadvertent contact with the microscope lens) and preventing it from drying out. In addition, mounting allows for long-term sample storage. Another important reason for mounting samples is to prevent photobleaching - the irreversible destruction of fluorophores that leads to a loss of signal.
Which mounting media should you use?
Mounting media fall into two main categories: water-based and solvent-based. Water-based mounting media allow immunostained samples to be directly applied to the glass slides. However, non-setting formulations usually require that a sealing material (e.g., nail polish or paraffin wax) be manually applied around the edge of each coverslip to prevent movement, which introduces an extra step into experimental workflows, while setting formulations eliminate the need for sealing but may incur a delay of up to 24 hours while the refractive index stabilizes. It is worth noting here that the refractive index of the mounting media should be matched to that of the glass slide to maximize image clarity and resolution. A major advantage of solvent-based mounting media is that they enable samples to be stored for years. But slides must be processed through a series of dehydration steps prior to mounting, and then left to cure overnight, which both takes time and creates extra solvent waste.
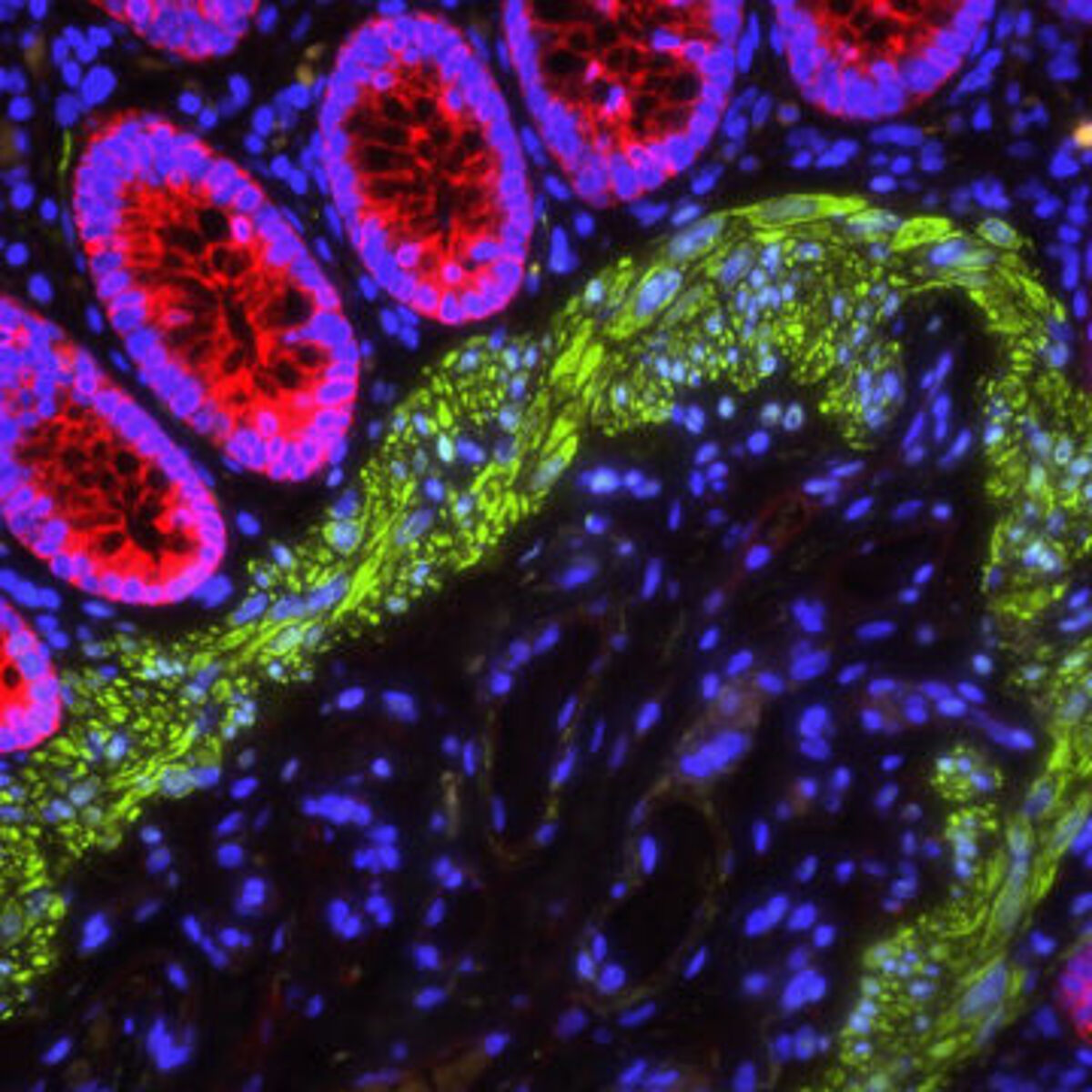
Introducing VECTASHIELD® and VectaMount® Express
VECTASHIELD® is a range of water-based antifade mounting media from Vector Laboratories that is optimized for IF applications. Available as setting and non-setting formulations, with a choice of counterstain or no counterstain, VECTASHIELD® enables samples to be viewed within just 1 hour of mounting. VectaMount® Express is a solvent-based mounting media that reduces the dehydration process from 30 minutes to just 2 minutes and has a shorter curing time (30 – 60 minutes) than established methods, meaning faster time to results.
LubioScience represents some of the most trusted brands in research and works closely with partners such as Vector Laboratories to offer high quality mounting media. Contact us today to discuss how we can support your project.
Supplier

Vector Laboratories
Vector Laboratories is recognized as a pioneer and market leader in immunohistochemistry, immunofluorescence, glycobiology, and bioconjugation products.
