Immunohistochemistry (IHC) is a technique used for visualizing tissue sections. Unlike other immunoassay techniques, it offers insights into protein distribution and relative abundance that can differ in conditions of health and disease.
How does multiplex IHC work?
Multiplex immunohistochemistry, also called multiple immunolabeling or multiplex immunostaining, follows the same principle as conventional IHC. An antibody is used to label a specific antigen within the tissue sample of interest (a cell surface receptor or an intracellular antigen, such as a structural protein or a kinase). A secondary antibody coupled to an enzyme such as horseradish peroxidase is used to visualize the antigen-antibody complex. In contrast to e.g. Western blotting, IHC uses substrates which precipitate at the site of action and thus show the precise localization of the antigen within the tissue.
Multiplexing IHC allows researchers to simultaneously label multiple antigens in a given tissue. This saves both time and valuable experimental material and enables comparison of the relative location of different antigens. In order to work properly, multiplex IHC relies on complex combinations of primary and secondary antibodies, as well as different enzyme/susbtrate combinations (see Figure).
How are samples prepared for IHC?
IHC is performed using tissue biopsy material that is either snap frozen in liquid nitrogen or formaldehyde fixed and paraffin embedded (FFPE) upon collection. Advantages of freezing are that it preserves enzyme activity and safeguards sensitive epitopes such as post-translational modifications. However, FFPE is more common as it maintains tissue morphology, provides easier handling, and allows long-term storage of samples at room temperature. More recently, a method known as CellCover has been developed that enables “liquid freezing” of samples in the refrigerator, providing a more practical alternative to established methods. After preservation, samples are sectioned and applied to glass slides. Frozen samples are then fixed with alcohol or acetone, while FFPE samples must undergo deparaffinization, rehydration, and – in many cases - antigen retrieval prior to immunostaining.
When is antigen retrieval necessary?
Antigen retrieval is the process of removing epitope-masking protein cross-links formed during formalin fixation. It is achieved via one of two methods - heat-induced antigen retrieval (HIER), which uses heat and an optimized buffer, or proteolytic antigen retrieval (PIER), which relies on enzymes such as proteinase K, trypsin, and pepsin. HIER is usually preferred due to being more controllable, but it is important to check antibody datasheets for the recommended antigen retrieval protocol.
What level of permeabilization and blocking does IHC require?
While detecting cell surface antigens is often straightforward, staining for intracellular antigens requires samples to be permeabilized with detergents or solvents. In either situation, non-specific binding sites must then be blocked and, where horseradish peroxidase (HRP) will be used for detection, endogenous peroxidase activity should be quenched to avoid unwanted background signal. In many cases, Fc receptor blocking may also be necessary, although engineered antibodies that circumvent this issue are now available.
Antibody considerations for IHC
A main consideration for IHC is whether to use monoclonal or polyclonal antibody reagents, or a combination of both. Monoclonals are highly specific, typically demonstrate consistent lot-to-lot performance, and are available in unlimited supply. Polyclonals recognize multiple epitopes and can provide signal amplification to aid the detection of scarce analytes. Recently, recombinant monoclonals have become increasingly popular since they address the issue of genetic drift that can affect antibody performance over time; they also provide ample opportunities for modification and engineering.
It is equally important to choose between direct or indirect detection. Direct detection uses labeled primary antibodies and offers the advantages of fewer protocol steps and greater flexibility for multiplexing. Indirect detection instead relies on labeled secondary antibodies, which provide signal amplification. For indirect detection, it is critical that cross-adsorbed secondary antibodies are used to avoid non-specific antibody binding to the sample.
Whichever immunostaining method is chosen, it is essential to select antibodies that have been validated for IHC and proven to specifically detect the target of interest. This information should be available on antibody datasheets, which should additionally state whether the validation was performed using frozen or FFPE tissue.
Why should you consider multiplexed IHC?
A major limitation of conventional IHC is that it allows detection of just a single marker per tissue section. Multiplexed IHC instead enables researchers to study cell types that can only be identified by a specific combination of markers or to visualize the distribution and abundance of several analytes relative to one another. Other advantages of multiplexed IHC are that it saves both time and precious sample material, which has driven its use for numerous clinical, translational, and basic research applications.
Challenges and considerations for multiplex IHC
When performing multiplexed IHC, careful antibody selection is crucial to ensure reagents will work in combination. For example, antibodies against cell surface proteins may be incompatible with staining protocols used for detecting intracellular markers, which involve fixation and permeabilization, while panel design is vital to avoid spectral overlap between different fluorophores. Experimental controls are also key and can be selected by referring to open-access resources such as UniProt, PAXdb, or proteinatlas.org for information about expected protein expression. To circumvent the need for laborious optimization, preformatted antibody panels are a cost-effective solution that can often save time and usually lead to stronger signals.
Examples of multiplex IHC stainings
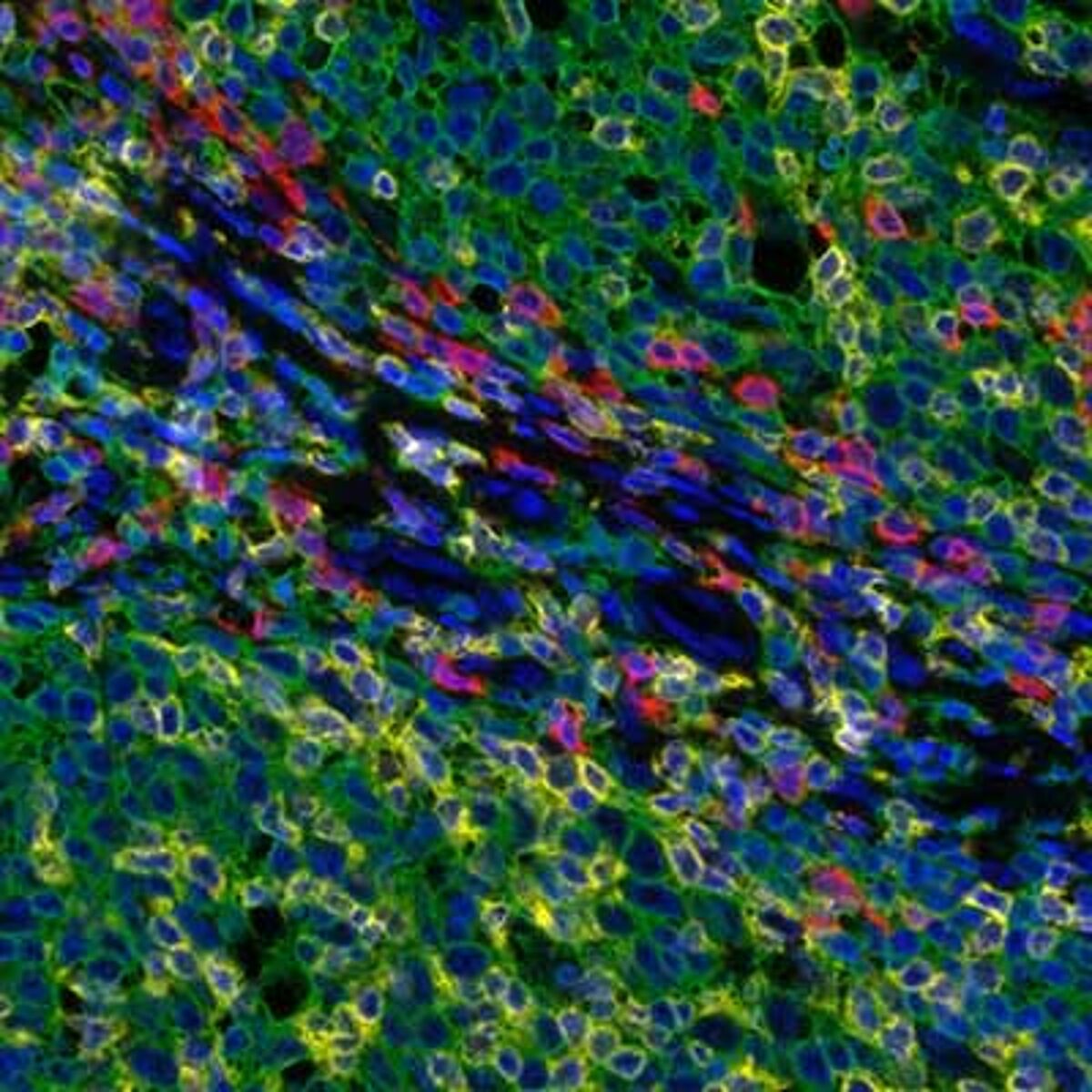
Detection of human CD3 (yellow), CD8 (red), and CD20 (green) in FFPE tonsil by IHC-IF. Rabbit anti-CD3e recombinant monoclonal [BL-298-5D12] (A700-016), rabbit anti-CD8a recombinant monoclonal [BLR044F] (A700-044), mouse anti-CD20 monoclonal [L26] (A500-017A). Secondary: HRP-conjugated goat anti-rabbit IgG (A120-501P) and HRP-conjugated goat anti-mouse IgG (A90-116P). Substrate: Opal™ 520, 620, and 690. Counterstain: DAPI (blue).
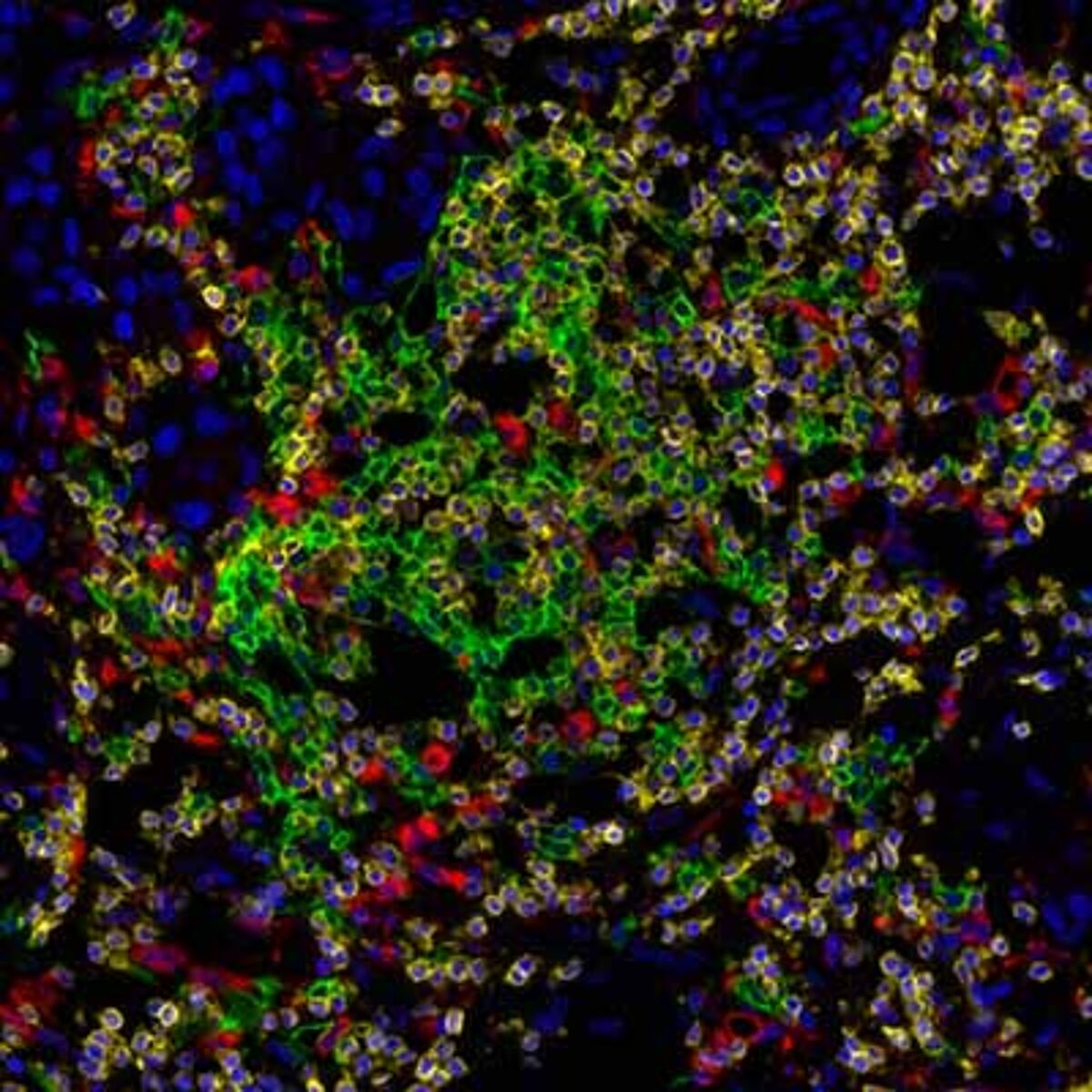
Detection of human CD3 (yellow), CD20 (green), and CD68 (red) in FFPE lung carcinoma by IHC-IF. Rabbit anti-CD3e recombinant monoclonal [BL-298-5D12] (A700-016), mouse anti-CD20 monoclonal [L26] (A500-017A), and mouse anti-CD68 monoclonal [KP-1] (A500-018A). Secondary: HRP-conjugated goat anti-rabbit IgG (A120-501P) and HRP-conjugated goat anti-mouse IgG (A90-116P). Substrate: Opal™ 520, 620, and 690. Counterstain: DAPI (blue).
Selected suppliers of multiplex IHC reagents

Bethyl Laboratories offers a wide range of primary and secondary antibodies which are validated for multiplex IHC and standard IHC applications. Bethly also offers PathPlex prevalidated antibody panels for IHC.

Proteintech offers a range of high quality, validated antibodies for IHC applications and also offers IHCeasy ready-to-use kits designed to provide all the antibodies and reagents needed to stain your tissue samples.

Atlas Antibodies is specialized in the production of antibodies for IHC applications. In order to guarantee specificity, all their antibodies are extensively validated in the applications they have been approved for: IHC, ICC-IF and/or WB.

Enzo’s superior MULTIVIEW® IHC kits allow efficient detection of multiple antigens in a single tissue sample. The kits include HIGHDEF® Chromogens for sensitive, crisp 2-color development. Biotin-free nanopolymer-based system for superior multiplex antigen detection.

CellCover is a non-toxic, ready-to-use fixative for immediate protection of biomolecules and cellular morphology. CellCover instantly fixes and stabilizes human and animal cells in their true state. Protein, RNA and DNA are all stored in their cellular context. Learn more.