What is the Nuclear Receptor Superfamily?
The nuclear receptor superfamily is a group of intracellular transcription factors that directly regulate gene expression in response to lipophilic molecules. These receptors are found in metazoan organisms such as nematodes, insects, and vertebrates. Nuclear receptors affect a wide variety of physiologic functions including development, reproduction, and metabolism and are associated with diseases such as Alzheimer’s, cancer, and diabetes.
How many receptors are in the nuclear receptor superfamily?
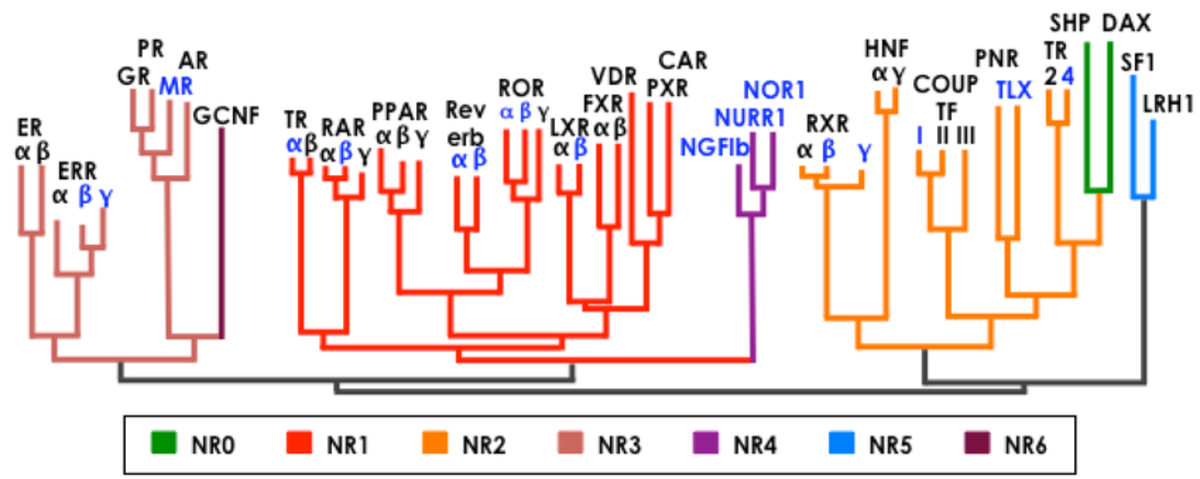
To date, over 300 nuclear receptors (NRs) have been found. A large number of nuclear receptors have been identified through sequence similarity to known receptors, but as of yet have no identified natural ligand and are referred to as ”orphan receptors.” Of the identified NRs, only 48 are found in humans with several of them being orphan receptors.
To organize the nuclear receptors in the nuclear receptor superfamily, an organizational nomenclature based on the NR’s phylogenetic tree was created by the Nuclear Receptors Nomenclature Committee in 1999. The form structure decided on is NRxyz, where x is the sub-family, y is the group, and z is the gene. For example, FXR is classified as NR1H4, meaning FXR is an NR in subfamily 1, group H, and is the fourth member of that group. There are seven different subfamilies indicated by NR1, NR2, NR3, NR4, NR5, NR6, and NR0. These subfamilies are classified based on the evolution of two domains found in the nuclear receptor superfamily, specifically the DNA binding domain (DBD) and the ligand binding domain (LBD). The exception to the rule is NR0, which are a subfamily of receptors that do not contain a DNA-binding domain, but instead bind to other nuclear receptors.
What domains are found in nuclear receptor superfamily members?
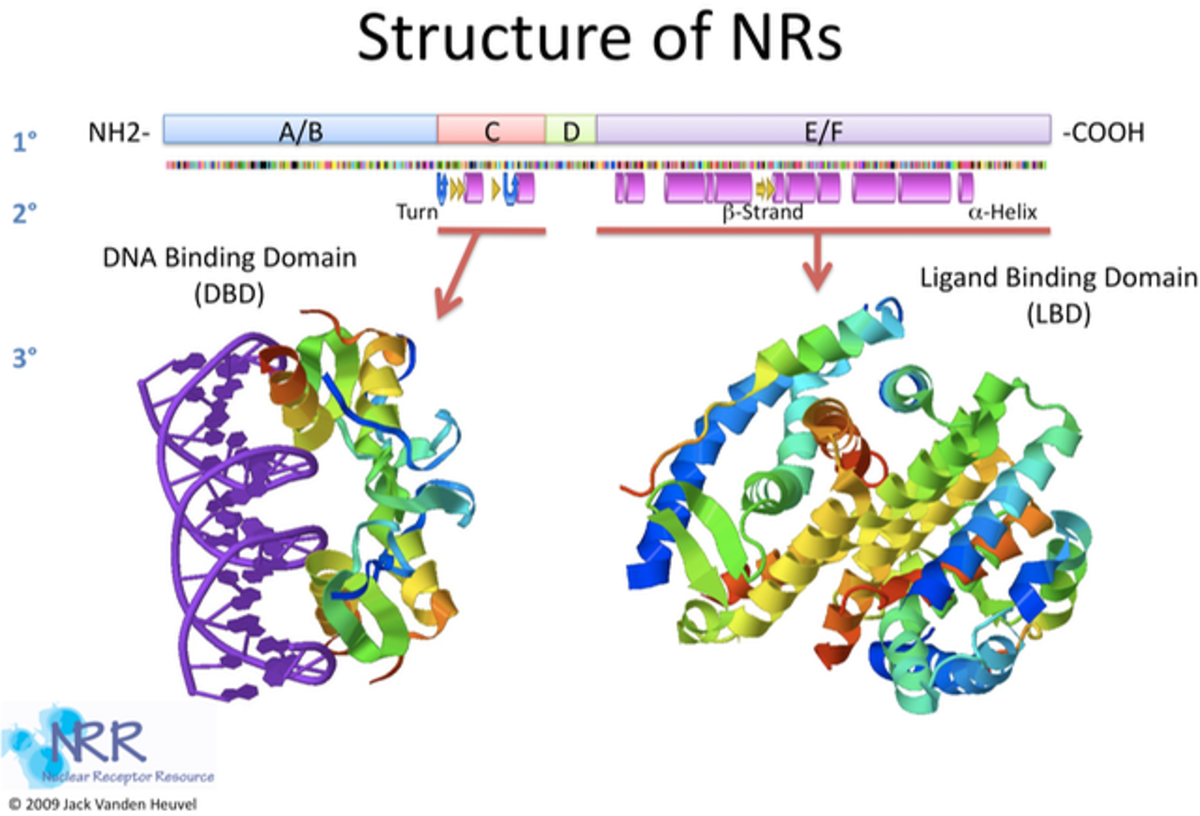
The nuclear receptor superfamily nomenclature is based on the nuclear receptors structure, but what is the common structure of the nuclear receptor superfamily? There are four main domains found in the structure of nuclear receptor superfamily members.
- The first domain is the N-Terminal domain or A/B domain which consists of the hypervariable region of regulation. The A/B domain’s sequence and length are highly variable between receptors and among receptor subtypes.
- The second domain is the DNA-binding domain (DBD) or C domain which is highly conserved with the signature motif of two C4 zinc fingers. The DBD binds to a specific sequence of DNA and as heterodimers, homodimers, or monomers, depending on the class of nuclear receptor.
- The third region is the hinge region or D domain which is flexible and contains the carboxy-terminal extension of the DBD. The hinge region appears to allow for conformational changes in the protein structure following ligand binding.
- The final domain found in the structure of nuclear receptors is the Ligand binding domain (LBD) or E/F Domain. The LBD varies substantially between nuclear receptors, but they all share a common structure of 11-13 α-helices organized around a hydrophobic binding pocket. Residues within the binding pocket determine whether the LBD will accept steroid hormones, retinoid compounds, or the host of xenobiotic ligands that affect receptor function. This section also contains the activation function 2 (AF-2) which is located at the extreme C terminus of the nuclear receptor.
Nuclear Receptor Superfamily in Drug Discovery
To better target receptors in the nuclear receptor superfamily, it is important to understand their structural and functional regulation. Research continues to define the biological niche of each nuclear receptor, as well as to understand overlapping pathways and functions. With nuclear receptors being involved in many disease states, being able to test for on- or off-target interactions with nuclear receptors can improve clinical outcomes by helping researchers make important decisions on which compounds of interest to move forward during drug discovery.
If you would like to learn more about individual nuclear receptors in the nuclear receptor super family or ways to test for on- or off-target interactions, visit INDIGO’s page. There you can find information on individual receptors as well as the assays that INDIGO offers for researchers to improve clinical outcomes.
References
Olivares, Ana & Moreno-Ramos, Oscar & Haider, Neena. (2016). Role of Nuclear Receptors in Central Nervous System Development and Associated Diseases. Journal of Experimental Neuroscience. 2015. 93. 10.4137/JEN.S25480.
Related Products from Indigo Biosciences
| Cat-No. | Item | Size | Price (CHF) |
|---|---|---|---|
| IB06001 | Human AhR Reporter Assay System | 1 x 96 wells | 1'453.00 |
| IB13001 | Human EGFR1 Reporter Assay System | 1 x 96 wells | 1'233.00 |
| IB17001 | EPOR Reporter Assay System | 1 x 96 wells | 1'453.00 |
| IB00401 | Human ERa Reporter Assay System | 1 x 96 wells | 1'453.00 |
| IB09001 | Human NF-kB Reporter Assay System | 1 x 96 wells | 1'453.00 |
| IB15001 | Human VEGFR Reporter Assay System | 1 x 96 wells | 1'233.00 |
| IB10001 | Human Nrf2 Reporter Assay System | 1 x 96 wells | 1'453.00 |
| IB00111 | Human PPARa Reporter Assay System | 1 x 96 wells | 1'453.00 |
| IB01001 | Human TRa Reporter Assay System | 1 x 96 wells | 1'453.00 |
Supplier

INDIGO Biosciences
Indigo Biosciences offer products and services focused on nuclear receptors. Learn more about INDIGO’s portfolio of assay kits and services.
More about Indigo Biosciences Shop for INDIGO Biosciences products