The Influenza virus challenges with a seasonal epidemic almost every winter in both the northern and southern hemispheres. The influenza virus has 4 types: A, B, C and D, but only A and B contribute to the annual ‘flu season’ and only A is known to cause flu pandemics (a global epidemic of flu disease). Influenza C generally causes mild illness in humans whereas influenza D mainly affects cattle and is not yet known to cause illness in humans.
With the potential to cause a large-scale human health crisis, influenza A has long been a subject of deep scientific research, intensified by the current COVID pandemic. It is now, even more essential than previously to understand circulating influenza subtypes, their global distribution and to enable diagnostic differentiation between influenza A, B, Coronavirus and Respiratory Syntactical Virus (RSV).
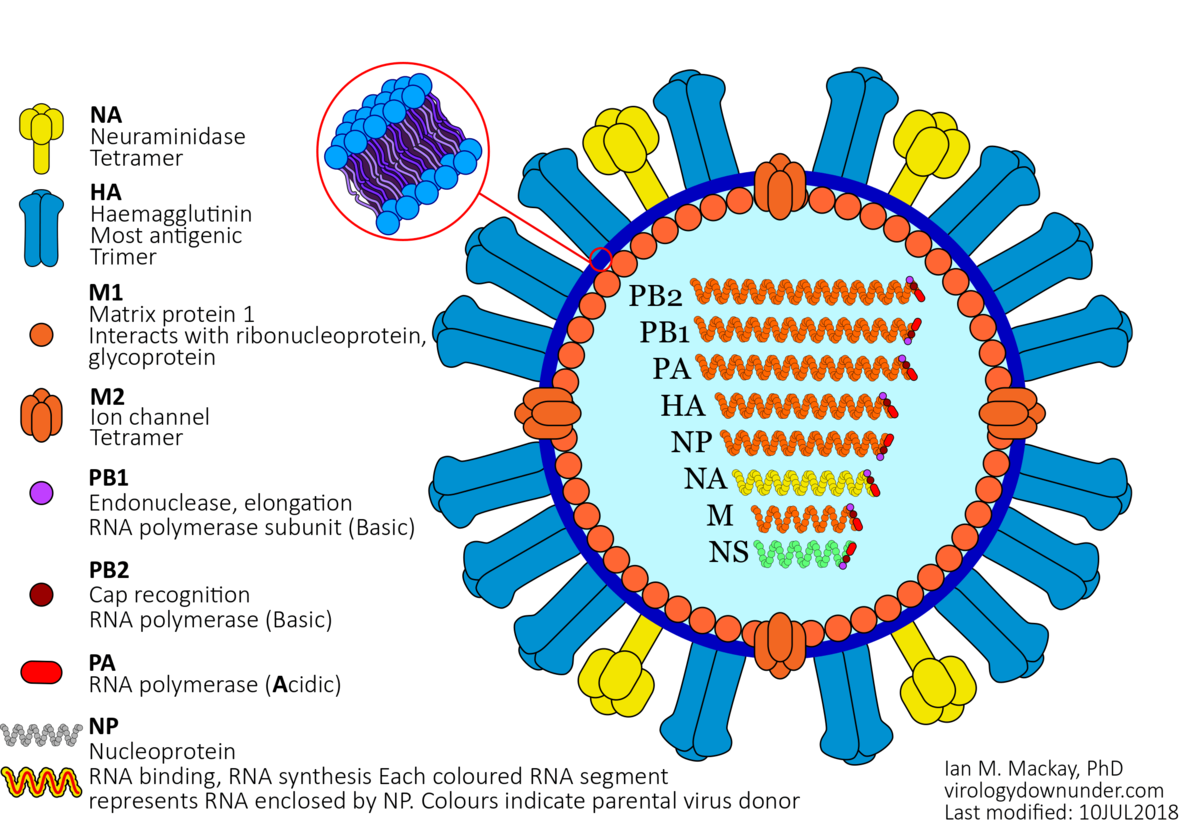
Influenza types are distinguished by differences in two major internal proteins – hemaglutinin (HA) and neuraminidase (NA). Influenza A is divided into subtypes based on the differences in these two proteins. There are 18 different HA subtypes (H1 – H18) and 11 different NA subtypes (N1 – N11). These HA and NA subtypes result in more than 130 influenza A subtypes, mainly identified in bird species. Within the Human population, H1N1 and H3N2 are the predominant circulating subtypes. Influenza A subtypes can be further broken down into genetic clades (groups) and sub-clades (sub-groups). The clade is defined by the nucleotide sequence coding for its HA protein.
By contrast, influenza B is classified into two lineages – B/Yamagata and B/Victoria before being further subdivided into clades and sub-clades. The genetic and antigenic properties of Influenza B virus changes more slowly than that of influenza A. It is common that both influenza A and B co-circulate globally, and multiple sub-clades of each virus can be present in the same geographic location within the same flu season.
Currently the circulating influenza A(H1N1) viruses are related to the spring 2009 H1N1 pandemic and sub-clades of this virus co-circulate with influenza A(H3N2) viruses. Recent years have seen flu B/Yamagata viruses circulating less frequently than flu B/Victoria on a global basis1
Naming Influenza Viruses
The rapid genetic changes observed within the influenza virus population requires an international naming convention to permit accurate study, tracking and vaccine development2.
The naming convention has the following components2:
- Description of the antigenic type of the virus (A, B, C, D)
- The host of origin e.g. swine, horse, chicken etc. If the originating host is human, it is omitted from the name.
- Geographical origin e.g. Wisconsin, Tokyo, England
- Strain number
- Year of isolation
This results in reference influenza strains named for example:
- A/Brisbane/59/2007 (Human origin)
- B/Florida/4/2006 (Human origin)
- A/Chicken/Guangdong/C273/2011 (Chicken origin)
- A/Egyptian goose/South Africa/AI1448/2007 (Egyptian goose)
Influenza Research reagents from Sino Biological
The likelihood of Influenza A and B to cause seasonal epidemics and even pandemics means ongoing research into this virus to better understand its genetic behaviour and to enable vaccine development is essential.
To facilitate this research and understanding access to a broad range of antibodies and antigens to the virus types and subtypes is essential. Sino Biological have developed a comprehensive catalogue of products:
- More than 2,500 research reagents: proteins, antibodies, genes, ELISAs
- More than 60 subtypes: Influenza AH1-H18, N1-N11, Influenza B
- More than 250 strains: Vaccine strains, HPAI strains, high attention strains, new strains
- Ten influenza virus antigens: HA (HA0, HA1, HA2, HA), NA, NP, M1, M2, NS1, NS2, PB1, PB2, PA
Sino Biological reagents are suitable for use in the following applications:
- Basic research
- Anti-virus drug development
- Vaccine development
- Virus detection research and diagnostic development
- Anti-virus antibody development
Influenza Virus Vaccines
Seasonal flu vaccines are the most effective way to prevent flu infection and a local epidemic. Each year, international surveillance data of recent isolates permits selection of dominant strains against which the next years vaccine is raised. Vaccines developed in recent years are now tri or quadrivalent and include one each of the dominant circulating H1N1, H3N2, B/Yamagata and B/Victoria strains.
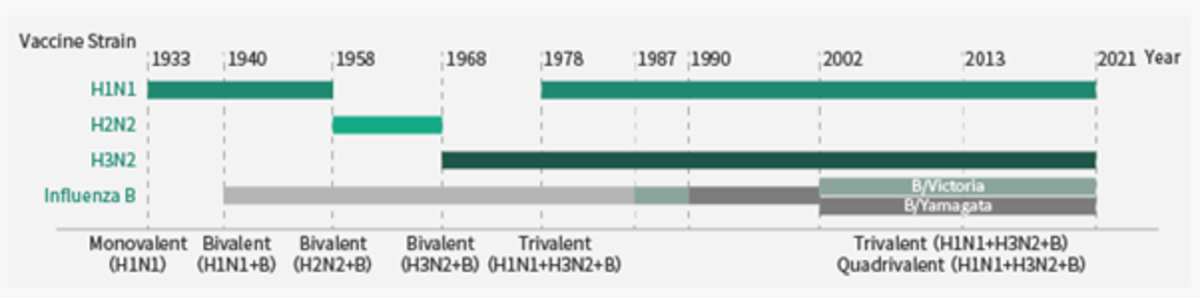
Sino Biological has developed recombinant antigens to most of the vaccine strains from 2015-2022 along with recombinant HA, NA and NP proteins from all WHO-recommended vaccine strains in recent years. These antigens can be used to analyse vaccine-induced antibody responses. They have also developed a large collection of monoclonal antibodies to the flue antigens, which help facilitate relevant assay development.
Selected influenza reagents
| Cat-No. | Item | Size | Price (CHF) |
|---|---|---|---|
| 11058-VNAHC1-200U | Influenza A H1N1 Neuraminidase / NA (H275Y) (Active) | 200 U | 431.00 |
| 40790-V08B-100UG | Influenza B (B/Washington/02/2019) Neuraminidase / NA (His Tag) | 100 ug | 588.00 |
| 40438-V08B-100UG | Influenza B (B/Florida/4/2006) Nucleoprotein / NP Protein (His Tag) | 100 ug | 588.00 |
| 86001-RM01-100UL | Influenza A Virus Hemagglutinin / HA Antibody, Rabbit MAb | 100 ul | 410.00 |
| 11675-MM03T-100UL | Influenza A Nucleoprotein / NP Antibody, Mouse MAb | 100 ul | 410.00 |
| 11058-MM07-100UL | Influenza A H1N1 (Swine Flu 2009) Neuraminidase / NA Antibody, Mouse MAb | 100 ul | 410.00 |
| VG11058-ACG-1UNIT | Influenza A H1N1 (A/California/04/2009) Neuraminidase / NA (Codon Optimized) Gene ORF cDNA clone expression plasmid, C-GFPSpark tag | 1 unit | 410.00 |
| VG40400-CY-1UNIT | Influenza A H9N2 (A/Hong Kong/2108/2003) Nucleocapsid protein / NP (Codon Optimized) Gene ORF cDNA clone expression plasmid, C-HA tag | 1 unit | 368.00 |
| VG40365-CF-1UNIT | Influenza A H9N2 (A/brambling/Beijing/16/2012) polymerase PA Gene ORF cDNA clone expression plasmid (Codon Optimized), C-FLAG tag | 1 unit | 399.00 |
| SEKA11684-5PLATES | Influenza A H1N1 (A/Puerto Rico/8/1934) Hemagglutinin / HA ELISA Pair Set | 5 plates | 420.00 |
| 40755-V08BL-300UG | Influenza B (B/Washington/02/2019) Nucleoprotein / NP Insect Cell Lysate (WB positive control) | 300 ug | 284.00 |
References
1 - https://www.cdc.gov/flu/about/viruses/types.htm
2 - Bulletin of the World Health Organization, 58(4):585-591 (1980)